All published articles of this journal are available on ScienceDirect.
Characterisation of Neonicotinoid Insecticides in the Cocoa-producing Owena River Basin of Nigeria by a QuEChERS Method Coupled to Liquid Chromatography-Tandem Mass Spectrometry
Abstract
Background:
Neonicotinoids (hereafter called “neonics”) are systemic insecticides used for the protection of agricultural crops. However, their dispersion in nature has been the subject of global concern due to reports of adverse effects on some living organisms. One of the applications of neonics in Nigeria is to protect the cocoa cash crop.
Objective:
Previous studies on pesticide-related pollution in Nigeria focused mainly on organochlorines, but research on neonics is sparse, and the knowledge gap needs to be filled. This work aimed at confirming the presence of four neonics, namely, imidacloprid, thiacloprid, acetamiprid, and thiamethoxam, within the Owena River Basin.
Methods:
Neonics were extracted from cocoa-growing soil, river water, and sediments by a modified QuEChERS method, followed by clean-up of the extractant by dispersive solid phase extraction and analysis by an optimized liquid chromatography/tandem mass spectrometry analytical procedure. The overall performance of these developed methods was then evaluated by set criteria.
Results:
The Limit of Detection (LOD) and Limit of Quantitation (LOQ) ranged from 0.0005 to 0.002 μg/g and 0.002 to 0.005 μg/g, respectively. The recovery for the four target analytes exceeded 75% across all matrices from laboratory-prepared samples. It was discovered that the average concentrations of three of the four neonics of interest in the individual media were: 10.34 nmol/g in cocoa-producing soil, 1.03 nmol/g in river sediment, and 1.08 nM (1.08 x 10-3 nmol/g) in surface river water. No imidacloprid was identified in any of these three environmental compartments.
Conclusion:
The concentration of neonics in the river water, i.e., 0.23 µg/L (230 ng/L), is identical to that of the maximum value recommended in the Canadian Water Quality Guidelines for the Protection of Freshwater Aquatic Life.
1. INTRODUCTION
1.1. Cocoa in the Nigerian Economy
The cocoa tree Theobroma cacao L. is a cash crop, and the beans are used as a feedstock for the production of cocoa liquor, cocoa butter, and cocoa powder. Therefore, cocoa is a raw material for the manufacturing of chocolate, with liquor and butter as its primary ingredients. In terms of global turnover, the market value of the bean was U.S. $9.94 billion in 2018 (Grand View Research, 2023), with the value of all cocoa traded globally being U.S. $12 billion (Kirsty, 2018). It has been predicted that the cocoa bean market size will expand to U.S. $16.32 billion by 2025 (James, 2019). The chocolate industry is known as the “100 billion-dollar” business, with sales totalling U.S. $113 billion in 2018, $118.6 billion in 2019 (UNCTD, 2019), and $106.6 billion in 2021 (Expert Market Research, 2022).
Cocoa trees are prone to various diseases, and one of the worst examples is that of an outbreak of the deadly (no known cure) swollen shoot disease in Ghana in 2019, which affected 16% of the crops; the impact of this disease on the yields of various cocoa clones was recently evaluated by Ofori et al. (2022). The price of cocoa is set by the International Cocoa Organization (ICCO). On 4th January, 2023, it was U.S. $2431.17 per metric tonne (International Cocoa Organization, 2023).
In the past four decades, the overall global trend in the production of cocoa beans has been continuously increasing despite various local setbacks. In the 1980/1 crop season, the world produced a total of 1.7 x 106 metric tonnes, and in (the pre-COVID pandemic) 2018/9 seasons, it was close to 4.9 x 106 metric tonnes (4.9 million metric tonnes), the highest production figure of all time (Statistica, 2019). Out of the 57 cocoa-producing countries in the world, the top five countries dominate the global supply chain, totalling 95% of the entire output, with the remaining 52 countries carrying only 5% (World Population Review, 2019). It is noteworthy that the economically top 4 African countries alone possess 68.1% of the market share, with Indonesia in Asia supplying a formidable 13.4% of all cocoa. The figures are listed in Table 1. For chocolate manufacturers, the uncertainty of the COVID-19 pandemic has made it important to monitor the developments in the cocoa-to-chocolate supply chain very closely.
| Country |
Annual Output (x Million Metric Tonnes) |
% of World Production = (x/4.9) x 100% |
| Ivory Coast | 2.034 | 50.8% |
| Ghana | 0.884 | 18.0% |
| Indonesia | 0.660 | 13.4% |
| Nigeria | 0.328 | 6.7% |
| Cameroon | 0.295 | 6.0% |
| - | - | Cumulative = 94.9% |
A year before the onset of the COVID-19 pandemic in 2020, Africa produced three-quarters of all cocoa beans worldwide and carried out a fifth of the grinding operations (UNCTD, 2019). As can be seen in Table 1, Nigeria alone accounted for 6.7% of the world’s harvest, with neighbouring Cameroon contributing 6.0% of all output. (The remaining 52 cocoa-producing countries accounted for just 5.1% of global production). Nigeria’s production dropped by 104 metric tonnes in the 2018/9 crop cycle compared to 2017/8 due to bad weather and ageing trees (Okojie, 2019).
Nigerian export is dominated by oil and gas but is followed immediately by its most profitable agricultural item, sesame seeds, with fermented cocoa beans in third place (Adesoji, 2019). Exported cocoa products (fermented and raw beans and butter) are approximately U.S. $0.75 billion annually, constituting 2% of all Nigeria’s exports. Other crops important to Nigerian agro-economics are peanuts, cotton, palm oil, corn, rice, sorghum, millet, cassava (manioc, tapioca), yams, and rubber (Ikenwa, 2020) and all require the care of integrated pest and disease management.
1.2. The Study Area
The cocoa crop is widely cultivated in southern Nigeria due to favourable soil and climatic conditions. The cocoa belt includes the States of Abia, Adamawa, Akwa Ibom, Cross River, Delta, Edo, Ekiti, Kogi, Kwara, Ogun, Ondo, Osun, Oyo, and Taraba, with Ondo being the top producer since 2005 (Oluyole, 2005). The River Owena runs along the major cocoa-producing sites of Ondo State in southwestern Nigeria, where insecticides are sprayed to control mirids, one of the major problems facing cocoa farmers in Nigeria. Another serious problem is that of the Black Pod Disease. It is arguably the most serious disease of cocoa in West Africa, especially in Nigeria. The disease is caused by a species of fungus called Phytophthora palmivora, a facultative parasite. These pests and diseases attack the leaves, stems, trunk, or pods of cocoa trees, contributing to substantial losses in cocoa production. It must be emphasized that routine maintenance, such as weeding, mulching, pruning, replacement of dead seedlings, and regeneration of old cocoa plants, are some of the production practices required for maximum productivity, in conjunction with the application of pesticides.
The Owena River basin is located at geographical coordinates, latitude 70 15′ (North) and longitude 50 5′ (East). The location of the sampling soil and surface river water was the Owena River at latitude: 7o 11’ 30” (North) and longitude: 5o 01’ 00” (East), in Owena Town at Ondo East Local Government of Ondo State (Fig. 1) (adapted from a publication of the African Development Bank Group, 2019). This is an environmentally sensitive area since the water of the River Owena has to be processed to potable standards for human consumption in this study area, comprising of Ifedore, Akure North, Akure South, Ondo West, Ondo East, and Idanre local government areas. Pollution of this river must be mitigated, but since it is possible that neonics in farmland may be leached into the river, it is necessary to survey the soil, river water, and sediment for the presence of neonics and gauge the level of contamination. Therefore, samples of soil, sediment, and river water were collected for analysis of neonics in June 2020, the beginning of the mid-crop season in Nigeria, which lasts until August. Recent research by Craddock et al. (2019) indicated the potential risk to human health posed by neonics, as these chemicals are now being discovered in water supplies and soil samples (Craddock et al., 2019).

1.3. Neonics as Insecticides and their Dispersion in Ecosystems
In 1985, Bayer patented imidacloprid as the first commercial neonic, and by the late 1990s, its efficacy was recognized worldwide, killing and controlling termites, beetles, locusts, and stink bugs alike. It was a time when organochlorines, organophosphates, and carbamates amounted to over 90% of the insecticides applied, but a new trend was developing. Although synthetic pyrethroids and other newer insecticides accounted for less than 5% of the number of insecticides used in 1997, they also covered about one-third of farmland areas subjected to insecticide treatment. Other neonics became available by 2013. Almost all corn planted in the United States was treated with clothianidin or thiamethoxam. By 2014, a third of American soybean acreage was planted with seeds treated with imidacloprid or thiamethoxam (Stokstad, 2013; U.S. EPA, 2021).
For their biocidal effect, use is made of the water solubility of neonics so that they can act as “systemic” insecticides, i.e., they can be coated onto crop seeds, adsorbed, and transported throughout the internal tissues of crops; some farmers spray them on foliage directly. Neonics can eliminate chewing insects as well as sucking pests, e.g., aphids, responsible for the spread of beet yellow virus on sugar beet. Farmers believe that treating seeds with the appropriate neonics can protect seedlings for up to ten weeks at the most vulnerable stage in their life cycle (McKay, 2019). This success has increased their popularity amongst farmers worldwide.
Once neonics enter insects, they begin to affect the nervous system. Neonics target the nicotinic acetylcholinesterase receptors (Moffat et al., 2016) in a similar fashion that nicotine binds to receptors on neurons in humans. The receptors are mostly found at the junctions, called synapses, where neurons are positioned in close vicinity and signal to each other. When a molecular entity binds to the receptor, it stimulates the nerve and creates an electrical pulse. This pulse is the information that tells the insect to crawl, fly, “think,” or “learn” (c.f., the acetylcholine molecule is a key neurotransmitter at the neuromuscular junctions of humans). Normally, an enzyme molecule comes along and deactivates or catabolizes the substance stimulating the nerve. Problems arise if the stimulant is a neonics. Neonic moieties are not catabolized or removed from the synaptic cleft easily. This high affinity to the receptor means that the nerve is continually stimulated.
A dose-response relationship may exist between neonics and insects. Normal neurophysiologic functions are first impaired, but as neurons are over-stimulated at higher doses, insects can become hyperactive, resulting in epileptic seizures and incapacitation of nerve cells. The authors of this paper did not disagree on the ecotoxicology of neonics, but recognized the prerogative to attribute bee death to varroa mites instead of neonics (Morrison, 2013). The dosages of neonics that bees encounter may or may not be high enough to kill them, and it is possible that bee colonies collapse because they do not return to their hives. For bees that survive after repeated exposure, it has been alleged that some get addicted to neonics, with the result that target nerve cells become more susceptible, thereby resulting in increased toxicity; one study also showed that bees’ genes could also be affected by neonics (Crall et al., 2018; Kenna et al., 2016; Arce, 2018; Evans, 2019; Colgan et al., 2019). Chan et al. (2019) performed an environmental risk assessment on hoary squash bees (Peponapis pruinosa) and other ground-nesting bees, with the risk posed by neonics in agricultural soil. The challenge for melittologists is to determine how affected individuals behave in ways that disturb the tranquillity of the order of a bee colony. Is it possible that the abnormal behaviour of intoxicated bees alerts the swarm to danger so that the entire colony emigrates? There are just 10 species of honey bees on Earth within the small monophylectic genus Apis (UNEP, 2018) and between 25,000 to 30,000 species of wild ones, although none live in Antarctica. (United Nations, 2019). Therefore, should we protect all of them, or some, and why? Another reason for conservation may be due to the rarity of the species (U.S. National Archives, 2016).
The theme of the discovery of neonics in a single targeted species of interest pervades the contemporary literature documenting the potential ecotoxicology of neonics. MacDonald et al. (2018) identified clothianidin and thiamethoxam in the carcasses of free-ranging wild turkeys (Meliagris gallopavo silvestris) in the province of Ontario, Canada. The turkeys might have ingested agricultural seeds coated with these neonics. The same researchers also discovered neonics-coated corn and soybean seeds in the gastrointestinal tracts of some birds. They envision the fruits of their research as “baseline data for Southern Ontario wild turkeys and provide a context for reference values in future analyses.” Interest in the effects of neonics on the fishery industry has also been rekindled recently. Main (2019) traced the plight of the Japanese eel in 1993. Adegun et al. (2020) identified neonics in six cultivable fish in the Owena River Basin by a QuEChERS/LC-tandem-MS method.
2. PERSPECTIVES IN WATER MANAGEMENT
Nigeria has a total surface area of about 923,770 km2, of which about 1.4% is covered with water. It has a large water resource potential, with annual yields estimated at 267 billion cubic metres of surface water and 52 billion cubic metres of groundwater. The country is drained mainly by the Niger Basin, the Lake Chad Basin, the South-Western Littoral Basin, and the South-Eastern Littoral Basin. The climate of Nigeria varies from semi-arid in the north to tropical and humid in the south. The average rainfall ranges from about 500 mm/year in the north to over 4,000 mm/year in the south. With approximately 1571 m3/per capita/year of renewable water resources, Nigeria should not be considered a water-poor country (FAO, 2016). However, Nigeria has experienced economic water scarcity, a situation which did not escape the notice of Falkenmark and was reported in three publications (Falkenmark, 1989; Falkenmark, 1990[i]; Falkenmark, 1990[ii]). Interestingly, Ibe-Lamberts (2012) noticed that, albeit the keen awareness of water problems in Nigeria, this has only a small effect on altruistic relief efforts on the part of transnational Nigerians.
In urban areas of the country, surface and groundwater are used as water sources, with treatment plants, distribution systems, elevated tanks, piped systems, house connections, and public standpipes, while the rural areas generally rely on groundwater with boreholes using hand pumps and protected wells.
In Nigeria, the Federal Ministry of Water Resources (FMWR) is responsible for developing national water policies, water resources management, and approving development projects supported by various agencies, including the 12 River Basin Development Agencies (RBDA), which are mainly responsible for the provision of bulk water supply for irrigation and urban water supply. The State Water Agencies (SWA) are responsible for developing and managing water supply facilities with the aim of providing mainly urban and semi-urban water supply, and in some cases, rural water supply and the Local Government Authorities (LGA) are responsible for the provision of rural water supply and sanitation in 774 local government areas around the country. There are currently two national standards for water quality in Nigeria, the National Environmental (Surface and Groundwater Quality Control) Regulations 2011 by the Federal Environmental Protection Agency and the Nigerian Standard for Drinking Water Quality 2015 by the Standards Organisation of Nigeria. However, none of this legislation mentions neonicotinoids by name.
Ondo state has a land area of 15,820 km2 and is located in the tropical rainforest zone of the country, with annual rainfall ranging from 524 mm to 2540 mm (African Development Bank Group, 2019). Ondo state has a population of 3.46 million, with 61% of people living in urban areas and 39% living in rural areas (Ibilewa et al., 2021). Ondo is an agrarian state, with 60 to 65% of its workforce engaged in agriculture. Moreover, Ondo is the largest cocoa-producing state in Nigeria and is responsible for up to 40% of cocoa export. (Ondo State Development Agency, 2018). Water supply in the state has declined over the years; most of the urban water schemes have become old and inadequate and are unable to meet the current demand (Oyebode and Omoya, 2019). Water supply and its access are low, with only about 9.6% of households having access to piped drinking water, while 54.2% get water from unprotected wells, ponds, and rivers (Ince et al., 2010).
Ondo State Water Corporation has 45 water supply schemes serving the inhabitants of Ondo state, with a combined capacity of 105,758 m3/day, which is about 70% less than the projected current demand (African Development Bank Group, 2019). The water supply scheme in the capital town of Akure draws raw water from the Owena River, which is treated in treatment plants with conventional treatment processes, such as screening, coagulation/flocculation, sedimentation, sand filtration, and chlorination (Oyebode and Omoya, 2019). Hence, it is of paramount importance to keep the Owena River clean.
3. THE TWIN-PRONGED OBJECTIVE OF THIS WORK
3.1. Ecological Risk Assessment
Environmental Impact Analysis (EIA) is the systematic identification and estimation of the potential impacts of proposed programs, policies, and legislative actions on the ecological and socio-economical components of the environment in totality. When the focus of an EIA is on the outcomes of the interaction between Homo sapiens and the natural environment of which they are a component, then it evolves into an Ecological Risk Assessment (ERA), which focuses on direct impact, requiring the setting up of the scope and boundaries of damage. While “risk” is defined as the probability that an undesired scenario will arise, an ERA attempts to characterize both arthropogenic and anthropogenic interferences on the health of natural resources, e.g., whether applied pesticides on a farm will pollute a nearby stream so severely that the capacity for treatment of this water for potability is exceeded. Therefore, an ERA appraises the behaviour of hazardous substances in the physical, chemical, and biological arenas. The physico-chemical environment includes major compartments, such as soil, geology, water resources, and air quality. The biosphere refers to humans (health and safety issues) and flora and fauna of the area of interest, including members of the plant and animal kingdoms (with exotic flowers, rare birds, bees, fish, and benthic macroinvertebrates often designated to be indicator organisms). Often, computer calculations of “Diversity Indices” are part of an ERA exercise, a parameter that quantifies meaningfully, in terms of ecosystem stability, the degree to which biodiversity is altered by the perturbations of many agents. The goal of all these assessments is to encourage thoughtful consideration of the total environment in planning and decision-making, arriving at actions that can lead to long-term developments which are beneficial and sustainable. An ERA (alone or as part of an EIA) often includes proposals to mitigate real and/or potential adversity in a final report. The present study can now be contextualized against cocoa farming in Nigeria. A sound strategy of integrated pest and disease control, which allows agriculture to thrive, can only commence with the collection of reliable field data.
3.2. Development of the Instrumental Method of Analysis of Neonics
This work also aims to demonstrate the ways in which a system of instrumental methods of analysis, namely, extraction of pesticides by a modified QuEChERS method and determination by LC-MS-MS, is capable of generating the field data required for comparison with the respective environmental quality standards (QuEChERS is a portmanteau word formed from the six adjectives “quick, easy, cheap, effective, rugged, and safe” (Anastassiades, 2003). These are characteristics ideal for processing large numbers of samples. This could be a preliminary step towards the most technically challenging activity of an ERA, predicting the types of impacts (qualitative depiction) and their magnitudes (quantifiable risks) on the various environmental components mentioned above in future endeavors of environmental protection. However, it must be verified that neonics are actually present in cocoa-producing soils, river water, and sediments of the Owena river basin.
Limited appraisals and reviews on the performance of QuEChERS in its many forms and modifications, and in conjunction with liquid chromatography/mass spectrometry combinations, have been provided by Schenck and Hobbs (2004), Dankyi et al. (2014), Abdel-Ghany et al. (2016), and recently by Zaidon et al. (2019). Soil and sediments are complex materials. Often, the physico-chemical properties of these substances differ from case to case, with each practical situation posing its own challenges in the analytical laboratory. The task of the chemical analyst is to develop a workable procedure accordingly. For this purpose, the literature can be consulted for solutions to the problems encountered.
4. MATERIALS AND METHODS
4.1. Laboratory Chemicals, Reagents, and Materials
All chemicals used for experimentation were of reagent grade. They also meet the specifications of the Committee on Analytical Reagents of the American Chemical Society (Tyner and Francis, 2017). Standards of pesticides of imidacloprid, thiacloprid, acetamiprid, and thiamethoxam (Fig. 2) as well as LC/MS grade water, HPLC grade methanol, and “Super-clean Tmenvi-CarbII/PSA” cartridges were provided by Sigma-Aldrich (UK), centrifuge tubes (50 mL) from Fisher Scientific (UK), sterile “millex” filter units (0.22 µm) from Merck Millipore (Germany), anhydrous magnesium sulphate, sodium acetate, ammonium formate, formic acid, and 3-chloroaniline (all of 98% purity) from Sigma-Aldrich (UK).
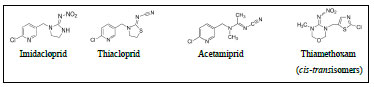
Standard stock solutions of the pesticides were prepared at a mass concentration mass of 1000 ppm. This was achieved by dissolving 0.01 g of each pure neonics standard in a mixed solvent of methanol and water (1:1 v/v) and diluting it in 10 mL standard flasks. The intermediate standard solutions were prepared at concentrations of 5 ppm. The spiking standard and internal standards were prepared from the intermediate standards at concentrations of 1.0 and 0.6 ppm, respectively. The calibration standards were prepared at seven to volume. Then, intermediate standard solutions were prepared at increasing concentrations: 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, and 1 ppm. The prepared standard solutions (stock, intermediate, calibration, and internal) were all stored in sealed PTFE containers in the dark to avoid any photolysis of the neonics.
4.2. Collection and Storage of Samples
Twenty core soil samples were collected under each cocoa plant canopy from the top 15 cm layer (0-15 cm) using a soil auger to form a composite sample. The composite soil samples were collected on aluminium foil, air dried at room temperature, and sieved through a 2 mm stainless sieve to remove coarse debris and stones (Asogwa and Dongo, 2009). Twenty sediment samples were also collected from 0 to 5 cm depths from the same spot from which the water samples were collected. The sediment samples were skimmed from the surface of the river bed and wrapped in aluminium foil, then air-dried and sieved through a 2.0 mm stainless steel sieve (Faraji et al., 2018). Ten water samples were collected from the river using pretreated Winchester glass bottles with Teflon-lined screw caps. The Winchester bottle stopper was removed just before sampling, and the bottles were rinsed thrice with river water and stored at 4oC in the laboratory prior to analysis. These samples were collected from the Owena river basin, Ondo State, South-Western Nigeria.
4.3. Extraction of Neonics from Spiked and Unspiked Soil, Sediment, and Water Samples
QuEChERS is a two-stage process. Stage 1 is a sample extraction step, and Stage 2 is known as a “dSPE clean-up” step.
4.3.1. Stage 1: Sample Extraction
Analytes were extracted from samples through the addition of an appropriate solvent and a blend of salts (AOAC, 2007). The salts enhanced the extraction efficiency and induced a phase separation between normally miscible organic solvents and water in the sample. The choice of the solvent system is of fundamental importance. Here, the leaching of neonics from solid matrices was optimized by observing the extraction efficiency of supposedly “blank” samples (i.e., samples with no pre-existing neonics) spiked at 1 ppm. A mixed solvent consisting of methanol (M), de-ionized water (W), and acetonitrile (A) was used for the extraction of the spiked solid matrices (soil and sediment). A non-aqueous solvent system consisting of methanol (M), hexane (H), and acetonitrile (A) was used for extracting spiked water samples. Each of the two types of mixed solvents was tested at four different mixing volumetric ratios in mL, namely, MWA ratio 1:1:1 v/v, 3:1:2 v/v, 3:2:1 v/v, 1:2:3 v/v and MHA ratio 1:1:1 v/v, 3:1:2 v/v, 3:2:1 v/v, 1:2:3 v/v, in order to ascertain the volumetric ratios, which allow the highest recovery of neonics. Acetonitrile plus 1% acetic acid, developed by the Association of Official Analytical Chemists (AOAC), was also used for extracting neonics from the spiked samples.
One gram of each sample was introduced into 50 mL centrifuge tubes, one tube per sample. Each sample was spiked with a 160 µL aliquot, which contained mixed standards of imidacloprid, acetamiprid, thiacloprid, and thiamethoxam. Together with the solvents, 3 g of MgSO4 and 3 g of CH3COO-Na+ were also added. This multiple-component mixture was shaken, mechanically vortexed, and treated in an ultrasonic bath at room temperature for 10 minutes. The mixture was further shaken on an orbital shaker for 20 minutes at 200 rpm and then centrifuged at 4500 rpm at 10°C for 3 minutes. From the supernatant fraction, 7 ml of the liquid was transferred to a 15 mL centrifuge tube, and a fresh portion of 1 mg of MgSO4 was added for the removal of excess water from the extracts. Extracts were centrifuged at 4000 rpm at 10 °C for 3 minutes. The extracted supernatants were collected in triplicate and combined for the next stage of the sample preparative procedure, that of “dSPE cleanup”. The mixtures of solvents with the best recovery of the analytes were used in the extraction of real samples from the Owena river basin.
4.3.2. Stage 2: dSPE Clean-up
Subsamples of the solvent extracted from Stage 1 were cleaned up through the use of dispersive solid phase extraction media, abbreviated in the literature as dSPE, prior to analysis by chromatography. First, a commercially available SPE cartridge designed for the cleanup of QuEChERS extracts (Superclean TMenvi-CarbII/PSA, Sigma-Aldrich) was conditioned with 6 mL of acetonitrile. An aliquot of the extract was transferred into it, and interferences, such as co-extractives, including sugars and fatty acids, were washed away from the sample matrix. The primary and secondary amines (PSA) were the base sorbent through which material exchange occurred during clean-up. Excess water and unwanted contaminants were also removed. The eluents were collected, and the cartridge was rinsed with deionized water, 5 ml of acetonitrile, and then with water again. A stream of nitrogen gas was then used to dry the cartridge. The extract was reconstituted to a volume of 0.75 mL (750 µL), followed by its filtration (by 0.22 µm membrane), and transferred to a 1.5 mL amber-colored vial. A volume of 0.45 mL of the 0.6 ppm internal standard (3-chloroaniline) was added to the reconstituted clean extracts, vortexed for a minute, and analysed by LC/MS/MS.
4.4. Optimisation of LC/MS/MS System
Separation of neonics by chromatography was performed on an “Agilent Technologies 1260 Infinity” High-Performance Liquid Chromatography (HPLC) system (from Santa Clara, California, USA) equipped with a triple quadrupole mass spectrometer (Lab X, 2020). The column used for the optimization exercise has a C18 packing of 5 μm particle size; the column dimensions were 2.1 mm (internal diameter) and 150 mm long. The temperature of the column was maintained at 31.95°C, and each individual injection volume was 10 μL. The autosampler temperature was set at 10°C. The washing liquid was a mixture of methanol and LC/MS grade water (1:1, v/v).
The elution gradient applied was “50% aqueous, 50% organic”. The phase labelled as “mobile phase A” was an aqueous solution of 0.1 wt. % NH4+(HCOO)- and 0.1 wt.% (HCOO)-H+. “Mobile phase B” was the organic phase with CH3OH as a solvent, in which 0.1 wt. % NH4+(HCOO)- and 0.1 wt. % (HCOO)-H+ were dissolved at 0.27 ml/min. (270 μL/min.) with a total run time of 15 minutes (including 3 min. for re-equilibration).
Optimisation of the mass spectrometer (MS) was performed by Agilent Technology 6400 in a positive ionization mode. The spray voltage of the electrospray ionization needle was 4500V. The vaporizer temperature was 420°C. The capillary temperature was 200°C, and the sheath gas pressure was 45 arbitrary units. Auxiliary gas pressure was 50 arbitrary units. The collision gas was argon, and the collision gas pressure was 1.2 mTorr. The neonics analytes and internal standards were detected in MS/MS conditions. The chromatographic acquisition run was programmed in selected reaction monitoring (SRM) mode. All ions were collected at a cell operating voltage of 7 V with a dwell time of 100 milliseconds. MS operating conditions were precursor ion mass, product ion mass, fragment mass, and collision energy [eV] for imidacloprid: 256.1 and 256.1, 209.0 and 175.1, 96 and 96, 14 and 18, thiacloprid: 253.0 and 253.0, 126.0 and 90.0, 103 and 103, 22 and 42, acetamiprid: 223.1 and 223.1, 126.0 and 90.1, 103.0 and 103.0, 18 and 38, thiamethoxan: 292.0 and 292.0, 211.1 and 131.0, 103 and 104, 8 and 20, and 3-chloroaniline: 128.0, 92.1, 96, 25. The characterisation of the masses was performed by the infusion of each of the four neonics after several injections. The collision energy was tuned for better fragmentation between masses 8 to 42, and the ions were well fragmented, with both precursor and product ions. The precursor and product ions were used for the quantification and confirmation of the analytes.
4.5. Performance Appraisal of the Proposed Method
The evaluation of the method was done by evaluating its precision by spiking the blank soil, sediment, and water samples obtained from the Hogsmill River close to Kingston University (London, United Kingdom, KT1 2EE). The matrices were spiked at 1 ppm level with standards of the pesticides. The percentage recovery of these analytes of interest describes how accurate the method would be in analyzing real samples. Linearity was determined at seven different concentrations, between 0.001 to 1.0 ppm, in triplicate. The calibration curves for the four compounds of interest were obtained by plotting the peak area ratio (response of the detector) against the concentrations of the corresponding calibration standards. The matrix effect was determined using the slope ratio of the calibrated curve. The expression for linearity of the calibration was expressed by the regression coefficient, r2 (Faraji et al., 2018). The limit of detection (LOD) is the lowest concentration or quantity of a component or substance that can be reliably distinguished with a specific analytical method. The Limit of Quantification (LOQ) is the lowest concentration of the analyte that can be detected and quantified within defined limits of certainty after replicate measurements are made on the blank and of known concentration. These parameters were determined by injecting ultra-low concentrations of the pesticides.
4.6. Quality Control and Quality Assurance
The quality control and quality assurance for this study include the use of a mixture of neonics spiked with an internal standard prior to extraction to ascertain accuracy. The precursor and product ions are shown in Table S1. Quantification was determined by plotting a calibration curve using seven calibration points. The average regression coefficient (r2) for the calibration curve was ≥0.990. Therefore, the precision of the proposed method was determined. The LOD and LOQ were measured based on signal-to-noise ratios of about 3 and 10, respectively. This is, in turn, based on the standard deviation of the response and slope of the calibration standards of seven replicate measurements. The values for these measurements, as well as the retention time of the analytes, are presented in Table S2. They also attest to the accuracy and precision of the measurements. The choice of 3-chloroaniline as the internal standard is owed to its similarity in molecular structure to the study compound; it is also chemically inert and exhibits a distinct elution time. Other quality control measures include repeatability and reproducibility, which were assessed from the injection of the mentioned standards on 6 repeated analyses on the same day.
For the quantification of analytes in spiked and unspiked samples, the linear regression equation of the simple form “y = mx + c” was used for the calculation of neonics residue concentrations in the investigated matrices. It can be calculated as:
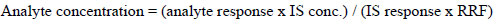 |
(1) |
Where RRF = relative response factor, IS conc = internal standard concentration, IS response = internal standard peak area, and analyte response = peak area of analytes
5. RESULTS AND DISCUSSION
5.1. Liquid Chromatography/tandem-mass Spectrometry Development
The optimized parameters for the LC/MS/MS procedure from a series of experiments to determine the best method for the analysis of target analytes are presented in Table S1. This was carried out to discover the conditions for the effective identification and quantification of these analytes. The methanol phase and the water phase (LC/MS grade water) were optimized with 0.1% v/v formic acid and 0.1% ammonium formate, respectively. Separation of the neonics was performed using the C-18 column, already described in Section 4.4 above. This was performed under different flow rates, column temperatures, and mobile phases before developing a steady and consistent separation method.
The time taken for the elution of the four analytes was less than fifteen minutes. The injected volume was 10 µL. The characterization of the masses was performed by the infusion of each of the four neonics: imidacloprid, acetamiprid, thiacloprid, and thiamethoxam, after several injections (Valverde et al., 2018). The ions were well fragmented with the precursor and product ions, as shown in Table S1. The precursor and product ions were used for the quantification and confirmation of the analytes, respectively (Valverde et al., 2018; Suganthi et al., 2018). The multiple reaction monitoring parameters (MRM) in this present study were in agreement with the study conducted by Suganthi et al. (2018) on the determination of neonicotinoid residues in sugar cane juice using LC/MS/MS. (Figs S1 to S6 for the ion chromatograms and calibration curves of the four neonics).
5.2. QuEChERS Method Modification
The extraction procedure for the samples was optimized with different solvent mixing ratios and analysed with optimized LC/MS/MS, already described in Section 4.4 above. The results of the recovery efficiencies showed that the mixtures of methanol, de-ionized water, and hexane with a volumetric ratio of 1:1:1 v/v (i.e., measured in 10 mL of each solvent, respectively) had the best recovery for all solid matrices. In the case of the methanol, hexane, and acetonitrile mixtures, it was the same ratio of 1:1:1 v/v that rendered the best recovery for the water matrix.
5.3. Method Validation
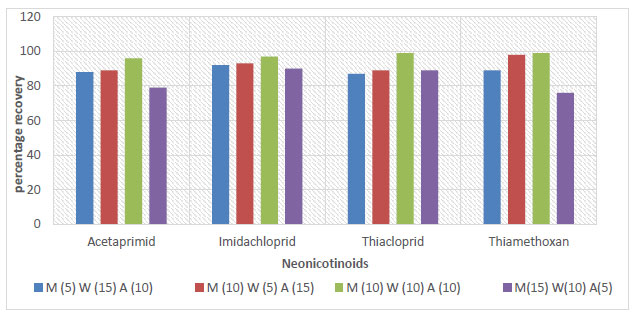
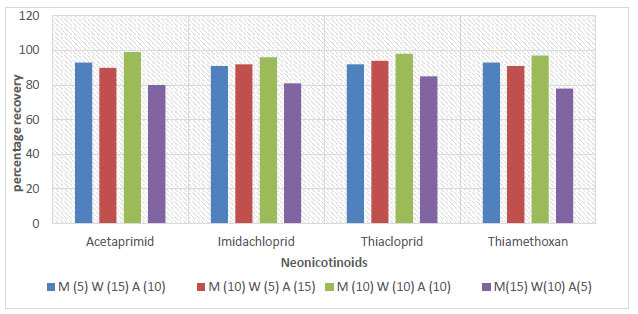
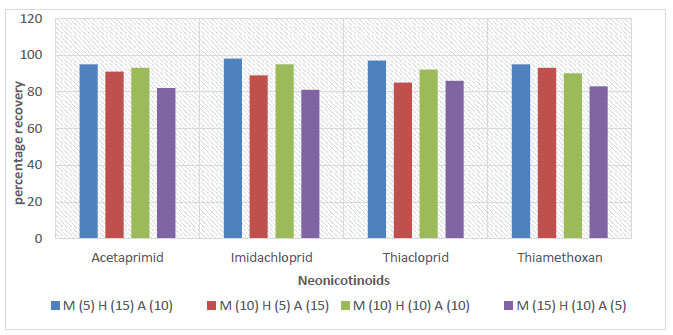
The evaluation of the parameters of the proposed method was based on some of the criteria stated in Section 4. The results of this evaluation are reported in Table 2. The recoveries of all spiked samples were within the range of recommended recovery limits of 70% - 120%, with a coefficient of variation ≤ 20% between replicates. The recoveries for the soil, sediment, and water samples ranged between 76 - 106%, 78 - 102%, and 81 - 98%, respectively, as shown in Figs. (3-5).
The recovery and reproducibility of the proposed method were reported to be sound. The recovery results of this study were in agreement with the recovery range reported in a previous study by Valverde et al. (2018). The proposed method was specific toward target analytes as there were no interferences due to the matrix effect. The routine calibration of the response of the detector to the concentration of the analyte, as discussed in Section 2, was made without error. Table S2 contains r2, LOD, and LOQ values. The regression coefficients r2 exceed 0.99 for all the neonics standards. The lowest LOD (of 0.005 µg/g) and LOQ (of 0.003 µg/g) encountered were both reserved for thiacloprid. LOD ranged from 0.0005 to 0.002 μg/g, and LOQ ranged from 0.003 - 0.005 μg/g.
| Neonics | Molecular Mass |
Average Mass Conc. (μg/g of Sediment) |
Average Amount Per Unit Mass of Sediment (nmol/g of Sediment) |
|---|---|---|---|
| Thiacloprid | 252.72 | 0.06 | 0.24 |
| Acetamiprid | 222.67 | 0.07 | 0.31 |
| Thiamethoxam | 291.71 | 0.14 | 0.48 |
| - | - | - | Total = 1.03 nmol/g |
5.4. Neonics in Cocoa-producing Soil Samples
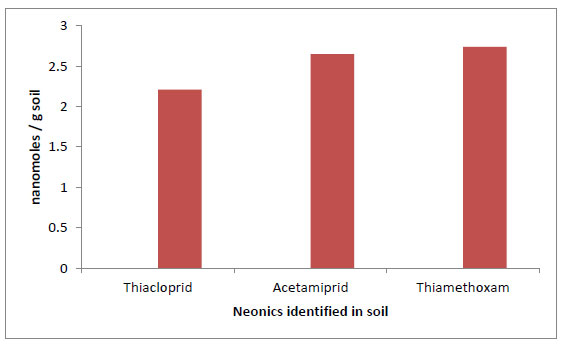
Only three neonics were found in the unspiked samples of cocoa-producing soil. They were thiacloprid, acetamiprid, and thiamethoxam (as shown in Table S3. In the mass concentration unit of µg/g, they ranged from 0.26 - 1.06, 0.31 - 0.78, and 0.29 - 1.56 with a mean concentration of 0.56 ± 0.35, 0.59 ± 0.25, and 0.80 ± 0.047 respectively. However, they are only mass concentrations, and to compare relative abundances, masses must be translated to moles (The S.I. unit of the mole expresses the amount of material with Avogadro’s number as a common basis). Here, the micrograms are changed to nanomoles (Table 3) and plotted in Fig. (6).
The frequency of occurrence of these neonics in the soil matrix indicates that farmers in the study area are currently using their active ingredients for cocoa pest control. Thiamethoxam and thiacloprid are currently approved insecticides for use in cocoa farms in Nigeria (Asogwa and Dongo, 2009). The variation in the level of these pesticides can be attributed to the frequency of their application, the amount applied, their physicochemical properties, and those of the soil (Asogawa and Dongo, 2009). However, the rate of degradation of neonics depends on the prevailing local conditions. Temperature, pH, moisture content, organic matter, and soil structure are all known to be important factors (Schaafsma et al., 2015). Moreover, soils act as sinks for applied pesticides. Neonics’ residues can be transported through the soil and leached into groundwater (Zhang et al., 2019), a source of drinking water in many countries. The trend in the contamination level of the cocoa-producing soil is thiamethoxam > acetamiprid > thiacloprid. It is possible that thiamethoxam is the most popular neonics used in the study area. Thiamethoxam is widely sold in the open market in Nigeria under the trade name “Actara-25WG”.
| Neonics | Molecular Mass | Average Mass conc. (μg/g of soil) |
Average amount per unit mass of soil (nmol/g of soil) |
|---|---|---|---|
| Thiacloprid | 252.72 | 0.56 | 2.21 |
| Acetamiprid | 222.67 | 0.59 | 2.65 |
| Thiamethoxam | 291.71 | 0.80 | 2.74 |
| Total = 10.34 nmol/g |
Investigations carried out by peers in the same field are worth mentioning as they contribute to the overall literature, which addresses the behaviour of neonics in the natural environment. However, the juxtaposition of diverse ecosystems and farming practices also highlights the difficulties of comparing data from different sources with a view of extrapolation from one scenario to another and hypothetical scenarios. For example, the mean value of neonics reported so far in this study, i.e., 0.56 - 0.80 µg/g (Table S3), was higher than the 0.0051 - 0.0345 µg/g range reported by Dankyi et al. (2014) obtained in Ghana, by 1 to 2 orders of magnitude. They also aimed to quantify neonics residues in soils from cocoa plantations using the QuEChERS extraction procedure and LC-MS/MS. In that study, only imidacloprid was detected in the soil samples; it could be a result of the frequency of its use in that area, unlike this current study area in which imidacloprid was not detected in any of the studied matrices or media.
In this study, neonics were found in all soil, water, and sediment samples, as shown in Fig. (5). This occurrence could be due to the soil being the primary source of aquatic and benthic pollution. The findings from this study were consistent with the range of solid matrix concentrations, i.e., 0.0051 - 0.0345µg/g, reported by Abdel-Ghany et al. (2016) from cucumbers and soil in cucumbers and soil in Qaluybiya, Egypt. Eight neonics were extracted by QuEChERS and quantified simultaneously by liquid chromatography coupled with tandem mass spectrometry. Clearly, the researchers were interested in ascertaining if safe levels of neonics were present in cucumbers for human consumption. Interestingly, metabolites of contaminants were not detected in this present study. Does that mean that biogeochemical and climate conditions in Qaluybiya favour faster degradation of neonics than the Owena river basin?
The nature and intention of each work of research on neonics are also different, albeit the fact that QuEChERS and LC-MS/MS methodology is employed in each of them. For example, Schaafsma et al. (2015) discovered that the concentration of total neonics residues in the leachate of corn fields “(per verbatim) increased six-fold during the first five weeks after planting and found in pre-plant levels seven weeks after planting. However, the concentrations in water sampled from outside the field were similar throughout the sampling period”. This is very useful information to have for long-term monitoring of the transport and distribution of neonics in the environment, made possible by the combination of QuEChERS and LC-MS/MS. Therefore, the focus of such research is to gain a longer-term understanding of the behaviour of neonics in the natural environment. Another good example of an attempt to elucidate the variation of neonics concentrations in natural bodies of water with time was made by Zhang et al. (2019), who reported the seasonal variation of five neonics in the sediment of the Pearl river estuary in the south of China. Similar research has been carried out by Bonmatin et al. (2019) and Main et al. (2014). It may also be inevitable that these instrumental methods of analysis have to be modified every time according to the realities and challenges of new situations.
5.5. Neonic Residues in Sediment Samples
The mean concentrations of neonics residues in the sediment samples (documented in Table S4) are listed in Table 3 and plotted in Fig. (7). For the residues of thiacloprid, acetamiprid, and thiamethoxan, these concentrations, in the unit of µg/g, ranged from 0.06 - 0.09, 0.04 - 0.12 and 0.08 - 0.20, with mean concentrations of 0.06 ± 0.02, 0.07 ± 0.04 and 0.14 ± 0.05, respectively.
These pollutants were also found in the soil samples. Their presence in the sediment is an indication that they could probably wash into Owena river through runoff and leaching, the two main pathways for land-to-water transport. The impact on benthic invertebrates by imidacloprid has been monitored (Chará-Serna, 2019).
These pollutants are also known to cause changes in swimming ability and respiratory disorders in Mayflies (Bartlett et al., 2018). By virtue of their relative immobility, the presence or absence of specific macroinvertebrates are good indicators of the absence or presence of certain types of pollution and their degree of severity, but almost no research work has been done on this aspect of river pollution as far as neonics are concerned. Nonetheless, the binding affinity of neonics for vertebrate receptors has been compared to that of invertebrates (Tomizawa & Casida, 2005). Zooplankton have also been known to be affected by thiamethoxam adversely (Lobson et al., 2018).
5.6. Neonics in Owena River Surface Water Sample
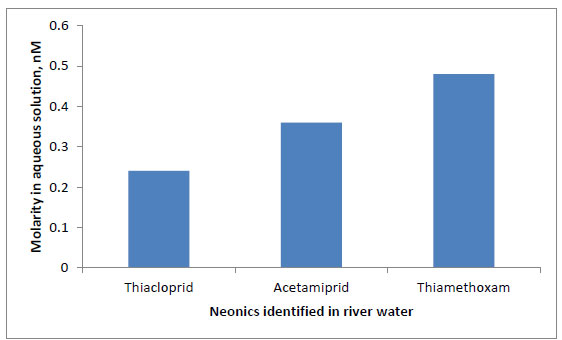
The concentrations of thiacloprid, acetamiprid, and thiamethoxan residues in the water samples (documented in Table S5, Supplementary Data, pp. 9) were noted in the unit of µg/dm3. These pollutants ranged from 0.03 - 0.08, 0.06 - 0.09, and 0.05 - 0.16, respectively, with mean concentrations of 0.06 ± 0.03, 0.08 ± 0.02, and 0.14 ± 0.05, respectively. The average molar concentrations are listed in Table 4 and plotted in Fig. (8).

Imidacloprid was not found in any sample collected for this work. This may be due to their non-approval for use in cocoa farms in Nigeria (Asogawa and Dongo, 2009). Similar studies by Mahai et al. (2019) on surface water neonics from the Yangtze River (China) and by Yi et al. (2019) on the presence of neonics in surface waters and sediments of Pearl river (China) also yielded interesting results.
| Neonics | Molecular Mass | Average Mass per dm3 of Water (Unit: μg/dm3) |
Average Molar Concentration (unit: nM) |
|---|---|---|---|
| Thiacloprid | 252.72 | 0.06 | 0.24 |
| Acetamiprid | 222.67 | 0.08 | 0.36 |
| Thiamethoxam | 291.71 | 0.09 | 0.31 |
| - | - | Total = 0.23 μg/dm3 (i.e., 230 ng/dm3) |
Total = 0.91 nM (i.e., 9.1 x 10-4 nmol/g H2O) |
Globally, water quality guidelines for the protection of aquatic life are a relatively rare entity. In 2007, Canada issued a federal freshwater guideline value of 0.23 µg/L (i.e., 230 ng/dm3) for imidacloprid (Canadian Council of Ministers of the Environment, 2007). Between 2012 and 2014, Strugel et al. (2017) collected samples across 15 water sites in the province of Ontario (Canada) to gauge the level of contamination by 5 neonics: imidacloprid, acetamiprid, clothianidin, thiacloprid, and thiamethoxam. By the time they published their findings in 2017, the figure 0.23 µg/L for imidacloprid had remained the only freshwater guideline available for any neonics being used in Canada. As a result, all measured concentrations of all neonics in their study were benchmarked to the same overarching value, 0.23 µg/L (230 ng/L), and documented as “supplementary information” in their paper. As of September 2019, there were no benchmark values specific to imidacloprid, acetamiprid, clothianidin, thiacloprid, and thiamethoxam recommended by the U.S. Environmental Protection Agency for assessing the risks and quality of aquatic life (U.S. EPA, 2022). Morrissey et al. (2015) surveyed the literature and collected a few guideline values ranging from 0.0086 to 35.0 µg/L. All of these values have to be understood and interpreted in the context of specific issues, both ecological and socioeconomic.
In the present work, the entire range of determined concentrations for all neonics (Imidacloprid, Thiacloprid, Acetamiprid, and Thiamethoxam) in the surface water of the Owena river fell in the range of 0 to 0.16 µg/L (the “zero” value is reserved for Imidacloprid since none was identified), which is below the Canadian benchmark value of 0.23 µg/L (i.e., 0.23 ppb). In Nigeria, the guideline value for maximum pesticide concentration in wastewater allowed for discharge into inland waters is set at 0.1 mg/L, i.e., 100 µg/L, or 100 ppb (UNEP, 1991).
Phenomenologically, there is also the mass transport of neonics between phases to be considered. No steady-state concentration of a pesticide in a naturally flowing system is beset by pollution episodes. The variation in the concentration of a pollutant in a river with time can only be modelled after prolonged observation of hydrological behaviour and much data gathering. Moreover, the rates of decomposition of a pesticide in soil, surface, river water, and sediment are not identical. In sediments, much less sunlight is available for photolysis, and the temperature is cooler. Intra-phase migration of pesticides in sediments is also plausible via Fickian diffusion or transport by bottom feeders. Moreover, while fish can swim away from pollution, benthic macroinvertebrates are domiciles in their habitat. Bees can get addicted to neonics until they are totally inebriated or die. All of these factors have to be taken into account when performing an Ecological Risk Assessment. The nature of the task of quantitative phenomenological modelling is challenging and colossal. These exercises have been attempted by Wood and Goulson (2017) and Utembe and Gulumian (2015).
5.7. Statistical Inference
The ANOVA test concluded that the p-values corresponding to the F-statistic values of all investigated matrices of soil, water, and sediment were higher than 0.05. This suggests that the means of the samples were not significantly different at the level of significance of 0.05. This was further confirmed by the Tukey HSD test, Scheffé multiple comparison test, and Bonferroni and Holm multiple comparison tests.
CONCLUSION
The use of biocides to control insects and diseases which plague cocoa plantations is paramount to the economic success of the agricultural sector in Nigeria. In the Owena river basin, neonicotinoid insecticides (“neonics”) are used to control mirids that attack the cocoa tree. Globally, neonics remain popular amongst many farmers, but the increased application of neonics to combat insect pests raises concerns about ecotoxicological effects. To be able to make sound decisions regarding field application through the processes of registration and issue of environmental health guidelines, awareness of the presence and distribution of these insecticides is important.
The neonics identified by this work were thiacloprid, acetamiprid, and thiamethoxam; all three were identified in soil, water, and sediment. Interestingly, imidacloprid, the most used neonics in the world cumulatively, as it was the earliest neonics to be commercialised, has not been detected (mentioned by Asogawa and Dongo, 2009). The concentration of each neonics in each soil, water, and sediment media was summed up to give an overall mean concentration of neonics present in the specific medium through statistical exercises: soil ~ 10 nmol/g; sediment ~ 1 nmol/g; water ~ 9 x 10-4 nmol/g. From a purely analytical chemistry perspective, it is clear that soil retains the largest amount of (three) neonics per gram of medium and is one order of magnitude above that of sediment and almost five orders of magnitude above water. One is tempted to infer that “soil is the most polluted of all ecological compartments”. However, it must be emphasized that the three compartments (soil, sediment, and water) are vastly different ecological systems in terms of intrinsic physical-chemical properties, exposure to sunlight, biodiversity of flora, fauna, and microorganisms, mass transport mechanisms of contaminants (diffusion vs. convection and bulk transport), impact of human activities, vulnerability to climate change, and rates of degradation of neonics in nature, i.e., the fate of each neonics is unique. If “pollution” is considered as the “wrong agent in the wrong place at the wrong time”, then perhaps it is not so adequately defined. Perhaps one can only study and manage specific instances of adversity.
By sheer serendipity, the concentration of neonics in the Owena river water, determined to be 0.23 µg/L (230 ng/L), is identical to that of the value recommended in the Canadian Water Quality Guidelines for the Protection of Freshwater Aquatic Life (Council of Ministers of the Environment, 2007) as a threshold of alertness to potential harm. The guideline was based on imidacloprid alone but has been used as a general guideline for all types of neonics detected in Canada. Can this be interpreted to mean that a finite probability exists that the health of a freshwater ecosystem will be compromised if the concentration of neonics exceeds the 0.23 µg/L value?
The combination of a modified QuEChERS method for the extraction of neonics from various matrices, followed by a clean-up step of the extractant by dispersive solid phase extraction (SPE) and determination by an optimized LC/MS/MS system has been an indispensable tool in this work. The development of this entire procedure has become part of the growing corpus of knowledge necessary for the detection/identification of ever-increasing novel molecular moieties embedded in complex multiphase samples.
In this study, the concept of Ecological Risk Assessment (ERA) was introduced as an intrinsic part of an Environmental Impact Analysis. Furthermore, data obtained by the instrumental method of analysis developed in this work were found to be fundamental to the recognition of risks posed to the total environment.
7. DIRECTIONS FOR FURTHER WORK
Owena river surface water is treated at the Ondo State Water Works. It will be interesting to compare the concentrations of neonics in the river water and treated water in future work. For example, a simple material balance on neonics will be informative as to whether the unit operations of the water treatment plant are effective in the removal of these insecticides. The World Health Organization (WHO) updates the list of contaminants to be tested in potable water and their acceptable mass concentrations (in mass concentration units of ppb and ppm) periodically. As part of the continuous effort to ensure that water is consumable by benchmarking against the latest standards, the testing of prescribed parameters in a reliable system of laboratory instrumental analysis is essential.
The molar balance for any contaminant, given the name “i” here, is as follows. For a treatment plant to be effective in its removal, the following condition of inequality must hold in a steady state:
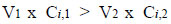 |
(2) |
Where:
V1 = volumetric flowrate of intake (e.g., m3/hr),
Ci,1 = molar concentration of contaminant i in untreated water (e.g., mol/m3),
V2 = volumetric flow rate of water leaving treatment plant (m3/hr),
Ci,2 = molar concentration of contaminant i in water leaving treatment plant (mol/m3).
Note that V1 will always be greater than V2 due to the evaporation of water and the formation of wet sludge. Also, ideally, Ci,2 = 0 after treatment, but this is rarely the case.
For sophisticated unit operations conducted in a continuous mode, such as the electro-oxidation of organic pollutants, V1 = V2. Therefore, for successful treatment, the following inequality must hold in a steady state:
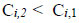 |
(3) |
Any method of treatment is totally inefficacious if the following equality holds:
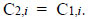 |
(4) |
Regardless of the choice of unit operations in a treatment plant, the input and output concentrations (C1,i and C2,i) are the parameters to be determined continuously by LC-MS-MS. Water treatment fails to achieve its objective when WHO guidelines and/or local statutory limits on water quality are not met. The close and continuous monitoring of the input and output concentrations of target contaminants through a water treatment plant is crucial all year round.
For the purpose of monitoring the fate of pesticides in the environment, whether in soil, river water, sediment, fish, or the cocoa bean, it is necessary to recognize the onset of crop seasons and the main natural events that occur during the season. In Africa, the cocoa harvest is not an activity constrained to a single and short time but is spread over several months. Harvesting can occur a couple of times a year. The timing of these harvests differs from one country to another, depending on the climate and the type of cocoa trees cultivated. In places with a clear demarcation of the wet and dry seasons, the main crop usually occurs about six months after the commencement of the wet season. The percentage differential between crops harvested in the main crop season and that of the mid-crop season also varies from country to country. For monitoring pesticide concentration in water, in Nigeria, the main crop season is from September to March, and the mid-crop season is from June to August (International Cocoa Organization, 2014).
Struger et al. (2017) carried out a 3-year survey of 15 water sites associated with agricultural activities in southern Ontario (Canada) from 2012 to 2014 with very interesting results quoted here per verbatim:
1. “Of the five neonicotinoids studied, imidacloprid, clothianidin, and thiamethoxam exhibited detection rates above 90% at over half the sites”.
2. At two sites, “the Canadian Federal freshwater guideline value for imidacloprid (230 ng/L) was exceeded” in about ¾ of the samples.
3. “For some watersheds, there were correlations between the occurrence of neonicotinoids and precipitation and/or stream discharge”.
4. “Some watersheds exhibited seasonal maxima in concentrations of neonicotinoids in spring and fall [autumn], particularly for those areas where row crop agriculture is predominant”.
These findings revealed the types of insecticides present in aquatic ecosystems in the geographical propinquity of Ontario farmlands, the extent they have polluted these natural bodies of water, the causative mechanisms of transport, and finally, seasonal variations of contamination levels related to agricultural activities directly.
The work of Struger et al. (2017) was also discussed because it was not markedly dissimilar in terms of motivation, experimental methodology, instrumentation, immediate investigative goals, and a longer-term vision of understanding human ecology on a deeper level than that of the present work. The difference is the period that spans the surveys, i.e., three years vs. “snapshots” taken of farmland and surrounding environments (during a doctoral program) and a project much larger in scale, with many more samples collected.
To the four discoveries listed above, a fifth insight can be added. Schaafsma et al. (2019) noticed that in south-western Ontario, where the main neonics applied are clothianidin and thiamethoxam, the “active ingredient may be abraded and lost in fugitive dust (during planting), and much of this active ingredient contaminate surface waters, exposing an aquatic organism to potential ill effects”. In fact, “only a small fraction of the neonicotinoid active ingredient is absorbed by the target crop when applied as a seed treatment, with the remainder entering the soil or degrading”. Schaafsma et al. (2019) determined “concentrations of neonicotinoids appearing in tile drains and open ditches around commercial maize fields around the planting time where neonicotinoid seed treatment had been used,” and they concluded that:
5. “For a no-observed-effect concentration of 0.3 ng/mL for thiamethoxam, there would be between a 1.6 and 100-fold margin of safety to mayflies in most streams if fugitive dust from pneumatic planters were properly mitigated”.
Gaining the knowledge expressed in points 1 to 5 above is both an example and a statement that Ecological Risk Assessment (ERA) can come to fruition. This should be the focus of further work in Nigeria, in collaboration with interested colleagues worldwide. Therefore, ecotoxicological research, which includes soil and sediment pollution and determining LC50 values of neonics to gauge toxicity to fish, can only lead to a deeper understanding of the fate of neonics on much larger scales. As demonstrated by this work, precise instrumental methods of analysis in a laboratory setting are the core of future research.
LIST OF ABBREVIATIONS
| ERA | = Ecological Risk Assessment |
| WHO | = World Health Organization |
| ICCO | = International Cocoa Organization |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available within the article. However, the samples are not available.
FUNDING
This work was funded by the Analytical Chemistry Trust Fund “Developing World Scholarship” of the Royal Society of Chemistry, grant number 19/600504/15, to Ayodeji O. Adegun.
CONFLICT OF INTEREST
Prof. James Barker is the editorial advisory board of The Open Environmental Research Journal.
ACKNOWLEDGEMENTS
The authors would like to thank Siamak Soltani-Khankahdani of Kingston University for his advice on GC/MS.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]


