All published articles of this journal are available on ScienceDirect.
Skin Spotting Variation Associated with Biometric and Reproductive Parameters in Naturalized Populations of Rainbow Trout, Oncorhynchus mykiss, from Southern Chile
Abstract
Background:
Skin pigmentation in fish is involved in various biological processes. In salmonids, the interactions of skin spottiness with biometric and reproductive parameters are mostly unknown, especially in naturalized populations influenced by different ecological factors.
Objective:
To associate skin spottiness variation with biometric and reproductive parameters in broodstocks of naturalized rainbow trout populations from southern Chile.
Method:
The number of dark spots below the lateral line was assessed in male and female broodstocks of rainbow trout from two reproductive seasons, years 2007 and 2012, and then this dataset was associated with biometric and reproductive parameters of the same individuals, using multivariate data analysis in the form of stepwise forward multiple regression.
Results:
Male body weight from year 2007 had a significant negative influence on the number of dark spots below the lateral line (P < 0.01), while the reproductive variables egg diameter and spawning time of females from years 2007 and 2012, respectively, had a significant positive influence on this parameter (P < 0.01).
Conclusion:
Our results indicate that there are male biometric parameters with a significant negative influence on skin spottiness. Our data also reveal that some reproductive parameters have a positive influence on skin spottiness. The identification of these reproductive parameters related to spottiness variation would reflect the reproductive quality of female broodstocks living in natural environments.
1. INTRODUCTION
Several studies have shown that skin pigmentation in vertebrates is a relevant character affecting different biological processes, such as the ornamentation of individuals, female mating preferences and the reproductive success of breeders in terms of offspring quality (Nordeide et al. 2013). In fishes, for example, its significance has been well documented in relation to the issue of sexual selection, as occurs in wild–type guppy males. In these fish, the male color pattern can determine the female mating preferences, since this character enhances the attractiveness of males during courtship (Kodric-Brown 1985, Houde and Endler 1990, Evans et al. 2004, Sathyan et al. 2013). In salmonids, a group that presents a characteristic species–specific pigmentation pattern (Colihueque 2010), studies have established that this character has a close relationship with developmental, reproductive and physiological processes. For example, some studies found that skin color intensity (i.e., redness) and body color are associated with offspring quality (Janhunen et al. 2011, Ramstad et al. 2010), and that the silver skin color is related to the smoltification capacity of different strains (Nichols et al. 2008). In addition, it has been reported that, in some species of this group, other skin pigmentation components, particularly the spotting variation, could be related with fish size (Aparicio et al. 2005) and with the different environmental features of the rivers inhabited by these populations. In the latter case, reflecting the role of skin pigmentation as a signal status that could determine the spatial arrangement of individuals along the river bed (Penteriani et al. 2015). Further studies have indicated that spotting variation in salmonids can be involved in behavior-related roles, such as stress responsiveness, given that highly spotted individuals are generally more resistant to stress than less spotted fishes (Kittilsen et al. 2009). Taken together, these data provide strong evidence that skin pigmentation in salmonids plays an important role in different biological processes and may provide clues to facilitate the analysis of these events.
The spottiness is a characteristic attribute of the skin pigmentation of rainbow trout, Oncorhynchus mykiss (Walbaum, 1792). In adult individuals of this species, this character is made up of conspicuous small dark spots, mainly distributed on the flank (above and below the lateral line), on the back, including the operculum and the dorsal and caudal fins (Ade 1989). The available evidence indicates that the spottiness is not a constant character in rainbow trout, given that it may vary within and between populations, e.g., in number both below the lateral line (Islam et al. 1973, Tack 1973) and on the back (Colihueque et al. 2011, 2014), or in size on the flanks (Kause et al. 2003). Thus, it is possible to find trouts within the same stock with a wide variation in the number of dark spots. For example, on the back, which may result in individuals with scarce or no spotting (< 0.5 spots/cm2), in contrast to others that can present a highly spotted skin (> 5.5 spots/cm2) (Colihueque et al. 2011). To date, the origin of this variation in the rainbow trout is unclear, however, it has been reported that the number of dark spots may vary in function of fish size and spawning time for the spottiness below the lateral line (Islam et al. 1973), and with age for the spottiness on back (Colihueque et al. 2011). In this species additional evidences also support the possible effects of genetic factors on the variation of the number of dark spots, either below the lateral line (Islam et al. 1973, Tack 1973) or on the back (Díaz et al. 2011). Most studies known to date on the analysis of the variation of the number of dark spots in rainbow trout have been carried out in cultured populations (Islam et al. 1973, Tack 1973, Colihueque et al. 2011, 2014), being their occurrence in natural or naturalized population of this species largely unknown. In natural populations of salmonid fishes, the evidence indicates a variation of this character is associated with biometric and environmental factors. For example, in brown trout the number of dark spots distributed below the lateral line is positively associated with fish size (Aparicio et al. 2005) and in graylings, the spotting level on flank presents a clear interaction with different environmental features of the river that they inhabit (Penteriani et al. 2015).
The rainbow trout was introduced to our country in 1905 (Golusda 1927). Currently, several naturalized populations of this species are found from northern to southern Chile inhabiting different river and lakes. In this body of waters, the rainbow trout has importance for recreational fishing purposes. The naturalized populations of rainbow trout inhabiting lakes from southern Chile, called Araucanian lakes, are adapted to particular limnic conditions given that these body waters present special environmental characteristics, such as a) glacial origin with volcanic influence in the soils, b) high deepness, c) thermal stratification, d) high in water transparency, and e) oligotrophic with very low salt/nutrient concentrations (Campos 1984, Arismendi et al. 2011). In addition, within these lakes, the naturalized populations of rainbow trout coexist with several native fishes and other introduced salmonids, most of them occupying the same habitat (Soto et al. 2006). Although some studies on the biology of naturalized populations of Araucanian lakes have been reported, such as those referring to the characterization of reproductive parameters and reproductive cycle (Wetzlar 1979, Soto et al. 2002), there have been no previous studies on their skin pigmentation. These populations provide an ideal opportunity to examine the relationship, for example, between the spottiness and reproductive parameters in nature to identify the factors that may contribute to spotting deviation. The spotting variation could be due to several factors, some of them being the heterozygosis of genes that control the trait, the interaction of individuals with different environmental features, the existence of different ecological pressures, sexual selection, and their correlation with behavioural and physiological traits, or a combination of the above. To assess whether the spotting level on the skin is associated with biometric and reproductive parameters of rainbow trout populations in nature, we investigated the relationship between the number of dark spots below the lateral line with different biometric and reproductive parameters in breeders of this species collected from a naturalized population.
This paper presents the result of the relationship assessed by stepwise multiple regression analyses between the number of dark spots below the lateral line with biometric and reproductive parameters by analysing male and female breeders from a naturalized population of rainbow trout that inhabit a lake from southern Chile. We analyzed breeders from two reproductive seasons to obtain a more complete view of the possible trends that could emerge among these parameters in nature.
2. MATERIALS AND METHODS
2.1. Fish Collection and Spawning
Sexually mature specimens were collected from different inlet streams of Calafquén Lake (39°31’S; 72°10’W, Villarrica district, La Araucanía Region, Chile), when the trouts carried out their reproductive run occurred from October to November, during the spawning season of the years 2007 and 2012. Next, they were reared at Chiguaico fish hatchery (Lican Ray City, Villarrica district) at a temperature that ranged from 7 to 8 °C with a constant flow of water at 10 L/s and a culture density of 15 kg/m3 in a raceway–type pond. The collected female and male specimens were measured for body weight (BW) and total length (TL) to the nearest 5 g and 0.1 cm, respectively. The condition factor (CF) according to the following formula was also calculated:
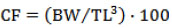 |
Means and ranges of biometric parameters BW, TL and CF of collected specimens are presented in Table 1. Ripe females were spawned individually and before handling they were anesthetized with a solution of benzocaine at 100 mg/L. Next, eggs were fertilized by a pool of diluted milt in Billard’s activator (Billard 1992) from four to six males. Fertilized eggs from individual females were incubated separately in plastic baskets within a shallow-trough incubator. Data was recorded for the following parameters: 1) Fertilization rate (FR) (%), 2) egg diameter (ED) (mm), 3) total fecundity (TF) (eggs/female), 4) relative fecundity (RF) (eggs/kg female), 5) eyed egg survival (ES) (%), and 6) spawning time (SPT) (number of days elapsed from the first day of the year until the spawning date of female). Means and ranges of the reproductive parameters FR, ED, TF, RF, ES and SPT of collecting females are shown in Table 2. Females and males were also photographed with a digital camera to register the number of dark spots on the skin. Once breeders were used in the reproductive process, all were returned live to the Calafquén Lake. Percentage data of FR and ES variables were arcsine-transformed prior to the analysis to better conform the assumption of normality. Rainbow trout specimens analysed in this study were captured during a restocking program of Calafquén Lake carried out from 2006 to 2012, since this lake had suffered a major decline in its population of rainbow trout, due to overfishing and poaching. To comply with the regulations of the Chilean fishing law, repopulation is performed from fingerlings produced by the breeding stock of the same basin. These broodstocks are captured during their spawning migration between the lake and the tributary streams. Calafquén Lake is representative of the rainbow trout population of the Araucanian lakes, since the introduction of rainbow trout was carried out in all of them from the same state hatcheries in the past.
| Parameter | Year 2007 | Year 2012 | ||
|---|---|---|---|---|
|
Females (N = 34) |
Males (N = 17) |
Females (N = 41) |
Males (N = 24) |
|
| Body weight (g) | 1794.7 ± 608.7 (845–3150) |
1074.1 ± 537.4 (495–2370) |
2076.7 ± 604.5 (658–3644) |
1532.6 ± 871.0 (424–4088) |
| Total length (cm) | 51.5 ± 5.9 (40.5–63.1) |
45.0 ± 7.6 (36.9–64.5) |
54.5 ± 5.8 (37–65.5) |
49.4 ± 8.4 (33.5–67.0) |
| Condition factor (CF) | 1.3 ± 0.2 (1–2.1) |
1.1 ± 0.1 (0.9–1.3) |
1.3 ± 0.1 (0.7–1.5) |
1.2 ± 0.1 (1.0–1.5) |
| Parameter |
Year 2007 (N = 34) |
Year 2012 (N = 41) |
|---|---|---|
| Fertilization rate (%) | 93.3 ± 11.2 (50–100) |
89.5 ± 9.2 (70–100) |
| Egg diameter (mm) | 5.4 ± 0.3 (4.7–6.1) |
5.3 ± 0.3 (4.5–6.1) |
| Total fecundity (eggs/female) | 2934 ± 889.9 (1102–4930) |
3456 ± 1298.1 (1332–7096) |
| Relative fecundity (eggs/kg female) | 1735.6 ± 539.4 (918.7–2930.9) |
1726.0 ± 568.9 (568.9–638.0) |
| Eyed egg survival (%) | 84.3 ± 16.3 (36.8–97.8) |
87.6 ± 10.9 (60.5–99.4) |
| Spawning time (days) | 300.2 ± 14.5 (278–330) |
286.5 ± 7.6 (274–302) |
2.2. Spotting Analysis
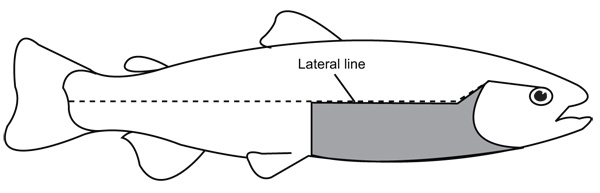
To perform the analysis of spottiness, the number of dark spots below the lateral line (NS-BLL) were counted in the area B of the body according to Qadri et al. (1959). This area corresponds to a rectangular zone of the skin located below the lateral line, in the posterior tip of the operculum and in the front base of the pelvic fin (Fig. 1). We selected this area given that it exhibits a high variation in number of dark spots on the skin in rainbow trout compared with others anteroposterior zones of this species according to previous studies (Islam et al. 1973). The NS-BLL were counted from digital photographs from the right side of the specimen. Only dark spots visible at naked eye were counted. Size and shape of the spots were not considered and fused spots were counted as one. The NS-BLL was log-transformed to achieve normality.
2.3. Statistical Analysis
The normality of variables was determined by the Kolmogorov-Smirnov (K-S) test. Differences between mean of numbers of dark spots were calculated with a two-way analysis of variance (2–way ANOVA) followed by Tukey’s multiple comparison tests to carry out post hoc pairwise comparisons of means (Sokal and Rohlf 1995). Forward selection stepwise multiple regression analyses were performed on NS-BLL with biometric (BW, TL and CF) and reproductive (FR, ED, TF, RF, ES and SPT) variables, as potential predictors to identify which variable, or combinations of variables, was most highly correlated with, and therefore possibly causally linked to, NS-BLL. Stepwise multiple regression is a useful tool for identifying combinations of independent variables that explain the most variation in another dependent variable (Sokal and Rohlf 1995). The criteria used for variable selection during forward stepwise regression were an F-statistic above 3.84 as entrance condition and an F-value of 2.71 as an exit criterion. The residuals of the final models were explored to verify the assumptions of normality, homogeneity and linearity. The absence of multicollinearity between independent variables was also verified through the variation inflation factor (VIF), based on the tolerance value of each variable, according to the formula VIF = 1/tolerance. When the value of the VIF was more than 5, the multicollinearity was considered serious and the variable was eliminated from the analysis (Ghani et al. 2010). In addition, to control the probability that a true null hypothesis had been incorrectly rejected as a consequence of multiple hypotheses testing, the entire data set was subjected to correlation analysis (Spearman’s rank correlation) between response and independent variables. This enabled us to apply a bootstrap procedure for correction of P-values, a re-sampling method that is recommended for correlated outcomes (Westfall and Young 1993). For this analysis, an α = 0.05 was used as the significant threshold. We also calculated Cohen’s d statistic to estimate the effect size, defined as the degree at which the null hypothesis is false (Cohen 1988). Cohen’s d statistic indicates that the greater the effect size, the greater the degree to which the phenomenon studied is present, i.e., this statistic estimates the magnitude of an effect of interest. Thus, d values between 0.5 and 0.8, and above 0.8 are considered to be medium and large effects, respectively. Statistical analyses were carried out using the STATISTICA program, version 5.1 (StatSoft Inc., Tulsa, USA, 1996) and the MATLAB R2010a program (The MathWorks, Inc., Natick, USA, 2010).
3. RESULTS
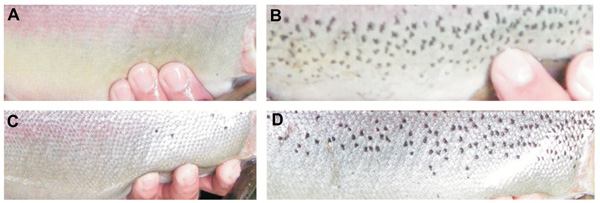
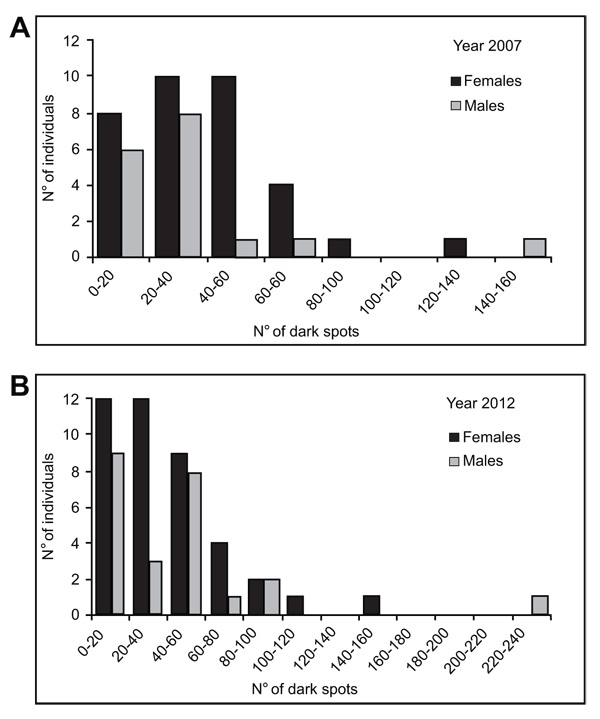
The number of dark spots varied widely in both reproductive periods, from 5 to 134 in females and from 0 to 146 in males from the 2007 year, and from 4 to142 in females and from 5 to 230 in males from the 2012 year (Table 3). Examples of male and female breeders of the 2007 year showing the extreme spotting variation are shown in Fig. (2). Moreover, this parameter showed normal distribution in males and females of both reproductive periods (K-S test, P > 0.10) (Fig. 3). Females from the 2007 year, displayed a higher mean number of dark spots than males (39.7 ± 26.1 and 34.6 ± 34, respectively), meanwhile the opposite was observed in females and males in the year 2012 (39.4 ± 29.8 and 43.9 ± 47.9, respectively) (Table 3). However, among these means, there were no significant differences (2-way ANOVA, F3/112 = 0.24, P > 0.05) either among sex within the same year or among sex between different years. It was noteworthy that the number of dark spots showed more variation in males than females in both years (males CV = 0.98–1.09 vs. females CV = 0.63–0.82). Indeed, in the year 2012 the number of dark spots in females exhibited less range (from 4 to142) than in males (from 5 to 230).
| Year 2007 | Year 2012 | |||
|---|---|---|---|---|
| Females (N = 34) |
Males (N = 17) |
Females (N = 41) |
Males (N = 24) |
|
| Mean ± SD | 39.7 ± 26.1 | 34.6 ± 34.1 | 39.4 ± 29.8 | 43.9 ± 47.9 |
| Range | 5-134 | 0-146 | 4-142 | 5-230 |
| Coefficient of variation (CV) | 0.66 | 0.98 | 0.76 | 1.09 |
Stepwise multiple regression for biometric parameters revealed a significant relationship, ranging from medium to large effects, in broodstocks during year 2007. Thus, results indicated a statistically significant relationship, and medium to large effects, between NS-BLL and BW for males (P < 0.01, d = 1.71) and NS-BLL and TL for females (P < 0.05, d = 0.73) (Table 4). BW in males presented a medium negative correlation with NS-BLL (β = -0.6496), while TL in females showed a weak positive association with NS-BLL (β = 0.3434). R2 indicated that the model explained 42.1% and 11.7% of the variability in the NS-BLL of males and females, respectively (Table 5). Bootstrap correction of P-values of Spearman correlation showed that only the relationship between NS-BLL and BW for males (rs = -0.5166, P = 0.0337, PBOOTS = 0.0371) was significant at P < 0.05. Thus, BW in males had the greatest influence on the response variable NS-BLL. For males 2012 no significant correlations were registered between NS-BLL and any biometric parameters (P > 0.05). In the case of BW a negative correlation was found (β = -0.2794), but without significance (exact P-value = 0.1860). Stepwise multiple regression for reproductive parameters revealed a significant relationship for females during the years 2007 and 2012 (Table 4). This analysis indicated the selection of only one of the six independent variables tested in the model, which had a significant association, and large effects, with NS-BLL that corresponded to ED for females in year 2007 (P < 0.01, d =1.0) and SPT for females in year 2012 (P < 0.01, d = 0.96). In both cases, the correlations were positives and medium between the response variable and the independent variable (β = 0.4487 and β = 0.4309, respectively). R2 indicated that 20.1% and 18.5% of variations in the response variable NS-BLL occurred because of changes in the independent variables ED and SPT, respectively (Table 5). Bootstrap correction of P-values of Spearman correlation showed that the relationships between NS-BLL and ED for females year 2007 (rs = 0.5328, P = 0.0011, PBOOTS = 0.0021), and NS-BLL and SPT for females year 2012 (rs = 0.4166, P = 0.0067, PBOOTS = 0.0113) were significants at P < 0.05. This result indicates that ED and SPT are important factors in the response variable NS-BLL . The equations of the fitted models were
Females year 2007:
NS-BLL = -0.9010 + 0.4461 x ED
Females year 2012:
NS-BLL = -3.8494 + 0.0186 x SPT
| Parameters/Groups | Model | Coefficients | SE | β | t-value | P |
|---|---|---|---|---|---|---|
| Biometric parameters | ||||||
| Males year 2007 | Constant | 1.9875 | 0.2075 | 9.5779 | < 0.001 (0.0000) |
|
| Body weight | -0.0006 | 0.0002 | -0.6496 | -3.3088 | < 0.01 (0.0048) |
|
| Females year 2007 | Constant | 0.6014 | 0.4458 | 1.3492 | > 0.05 (0.1868) |
|
| Total length | 0.0178 | 0.0086 | 0.3434 | 2.0681 | < 0.05 (0.0468) |
|
| Reproductive parameters | ||||||
| Females year 2007 | Constant | -0.9010 | 0.8529 | -1.0564 | > 0.05 (0.2987) |
|
| Egg diameter | 0.4461 | 0.1571 | 0.4487 | 2.8400 | < 0.01 (0.0078) |
|
| Females year 2012 | Constant | -3.8494 | 1.7923 | -2.1478 | < 0.05 (0.0380) |
|
| Spawning time | 0.0186 | 0.0063 | 0.4309 | 2.9821 | < 0.01 (0.0049) |
|
| Parameters/Groups | R | R2 | Adjusted R2 | SE | F | P |
|---|---|---|---|---|---|---|
| Biometric parameters | ||||||
| Males year 2007 | 0.6496 | 0.4219 | 0.3834 | 0.3736 | F1/15 = 10.948 | < 0.01 (0.0048) |
| Females year 2007 | 0.3434 | 0.1179 | 0.0903 | 0.2938 | F1/32 = 4.2772 | < 0.05 (0.0468) |
| Reproductive parameters | ||||||
| Females year 2007 | 0.4487 | 0.2013 | 0.1764 | 0.2796 | F1/32 = 8.0656 | < 0.01 (0.0078) |
| Females year 2012 | 0.4309 | 0.1857 | 0.1648 | 0.3005 | F1/39 = 8.8930 | < 0.01 (0.0049) |
4. DISCUSSION
Skin pigmentation in fishes has been related to biological and environmental parameters to try to understand if the variation of this character, either within or among populations, has a biological significance. To date these studies have been able to clarify the participation of this phenotype in several processes, for example, in predation avoidance (Endler 1980), in female mating preferences (Kodric-Brown 1985, Houde and Endler 1990, Evans et al. 2004), in phenotypic plasticity that may facilitate invasions in nature (Westley et al. 2013), in cryptic camouflage (Donnelly and Dill 1984, Donnelly and Whoriskey 1993), in status signals related to the spatial arrangement of individuals along the river bed (Penteriani et al. 2015), and, also, in stress response of individuals (Kittilsen et al. 2009). In spite of this progress, few studies have been conducted on the role of skin pigmentation in relation to the reproductive success of broodstocks, although the scarce data available on this issue suggest its possible role in spawning displays and reproductive success (Wedekind et al. 2008, Janhunen et al. 2011). This study contributes to understanding the influence of skin pigmentation, particularly skin spottiness, on reproductive and biometric patterns observed in two naturalized populations of rainbow trout. Through this analysis, we provide new data on the possible trends that could emerge among these parameters in nature, in which particular intrapopulation interactions may have taken place in response to different ecological factors.
In this study, we support that below the lateral line (area B) the number of dark spots is also a variable character in naturalized populations of this species from southern Chile. Thus, for example in the 2007 stock, this parameter varied from 4 to 142 and from 5 to 230 dark spots in females and males, respectively. As far as we know, to date no data on naturalized population from Chile and other countries has been published on this character to compare our results. However, taken into account the data available for the cultured population of this species, our results have a good concordance. Indeed, Islam et al. (1973) reported hatchery populations from Japan with a number of dark spots in the area B ranging either from 6 to 338, or from 12 to 316. In addition, our data also agree with reports from the other cultured population, as is the case of a German population where this character varied from 0 to 250 (Tack 1973). In summary, our data along with previous studies indicate that the number of dark spots below the lateral line of the rainbow trout is mostly a polymorphic character, a phenomena that are displayed by cultured and naturalized populations of this species.
The variation in the number of dark spots in the naturalized population of rainbow trout studied here is an interesting result that will require more in depth investigation to clarify their origin. However, this variation appears to be not sex-linked given that males and females analyzed in this work from both reproductive periods did not present significant differences in this parameter. Other factors, mostly studied in cultured populations of this species, although not analyzed in this work, may involve the effect of genetic, environmental and even, behavioural factors. For instance, the strain-specific variation in the number of dark spots below the lateral line (Islam et al. 1973), and the transmission of this character to the offspring in experimental crosses of progenitors with contrasting number of dark spots (Tack 1973), represent findings that support the effect of genetic factors in rainbow trout. In line with these evidences, more recent data obtained in this species also indicate the effect of a polygenetic determination for the dark spotting on the skin, whose heritability (h2) could reach a value ranging from h2 = 0.16 (Díaz et al. 2011) to h2 = 0.45 (Kause et al. 2003). In addition, the action of environmental factors on the spotting level in rainbow trout would also occur based on the evidence documented in other salmonids. Particularly, in the brown trout, where it has been demonstrated that the variance of the number of dark spots below the lateral line could be subjected to a significant environmental effect (Blanc et al. 1982), and in graylings, whose spotting level in the flank is positively associated with particular environmental features of the river, such as water velocity, water depth and water transparency (Penteriani et al. 2015). Other studies even support the effect of behavioural factors, given that in rainbow trout and Atlantic salmon the variation in spots number is related to the stress response of individuals (Kittilsen et al. 2009), being this capacity, higher in spotted fish than in non-spotted fish.
In females of the year 2007, we found a weak positive association between the number of dark spots below the lateral line and total length. This pattern is discordant with previous studies since, in rainbow trout, a clear relationship among these variables has been described for specimens above a certain size (> 30 cm) (Islam et al. 1973). Our finding also disagrees with reports from other salmonid fishes, such as the brown trout, since total length in this species is significantly associated with the number of dark spots located below the lateral line (Aparicio et al. 2005), with those distributed in the ventral zone (Blanc et al. 1982), and with the total number of red spots on the body (Kocabas et al. 2011). In contrast, the males analyzed in this study, especially those of the year 2007, exhibited a strong inverse relationship between both parameters, i.e., larger specimens tend to have a lower number of dark spots below the lateral line than small ones. It is possible that this association may be related to the different development stages or ages of males analyzed if we consider that the spotting level decrease across the development. However, the available evidence in the rainbow trout indicates that on the contrary, this interpretation would not be consistent given that the spotting level of this species increases steadily across the development, such as have been observed at juvenile stage (Colihueque 2011). Therefore, it is likely that other factors could be involved in the origin of this pattern, being environmental factors an interesting hypothesis to be considered in the light of the evidence obtained in other salmonids. For example, in natural populations of graylings, Penteriani et al. (2015) have supported the interaction of the spotting level on flank with different environmental features of the river where they inhabit, such as water velocity, water depth and water transparency. Indeed, individuals with high spottiness level exhibit a positive and significant association to fast waters or with greater depths. Interestingly, these interactions also reveal that the spotting level may constitute a status signal related to the spatial arrangement of individuals along the river bed. Although further analyses on this issue are required to clarify the possible effects of environmental factors on the spotting level in naturalized populations of rainbow trout, in our case it should be noted that specimen analyzed were collected at spawning season from the small inlet streams of Calafquén Lake during their migration period. As it has been observed in this species (Knapp and Vredenburg 1996), the spawning behaviour is a complex process that involves the interaction of spawning females with particular characteristics of the river to construct their redds, such as water velocity, water depth, and substrate size. Moreover, in this process the interaction of females with males is also important for fertilization success, especially, if we keep in mind that in natural conditions ripe males are 3-9 times more abundant than ripe females (Knapp and Vredenburg 1996). Thus, it is possible that the spotting variation of males that is negatively associated with size may constitute some class of signal to address the interaction by spawning females.
The relationship between skin color and reproductive parameters in rainbow trout has received little attention. In vertebrates, including fishes, this approximation has been analyzed in the context of the effect of female ornamentation on offspring quality, and therefore, as a factor affecting fitness (reviewed by Nordeide et al. 2013). These studies indicate that different vertebrate ornamentation attributes (e.g., plumage coloration, size of black feather spots, skin color and body color) are related positively or negatively at different levels; for example, with the survival and body mass of the offspring and with the size, mass and color of eggs. In particular, the effects of spotting level on fitness were well documented in Rana pipiens several years ago (Merrel 1972). It has also been observed that a particular female ornamentation may affect the female’s own reproductive parameters, such as spawning time and fecundity (Roulin et al. 2001, Janhunen et al. 2011). In our case, results indicating that the existence of some reproductive parameters in female rainbow trout broodstocks, particularly egg diameter and spawning time, were significantly associated with the number of dark spots below the lateral line, suggest that this skin color attribute may be linked with female fitness. Thus, the spotting level could be interpreted as an indicator that reflects the reproductive quality of females, especially if we take into account that egg diameter (Brooks et al. 1997) and spawning time (Siitonnen and Gall 1989, Quinn et al. 2002) are important traits for salmonid reproduction. In addition, since our study analyzed a naturalized population of rainbow trout, it is possible that ecological factors may be involved in the origin of this relationship. This possibility is not unlikely, in view of ecological studies on different fishes which indicate that greater reproduction success in females under natural conditions appears to depend on egg size, since larger eggs provide survival and growth advantages to the offspring during their first few days of life (Brooks et al. 1997). In addition, our findings on the association between spottiness and spawning time, agree with other previous studies that reveal the existence of this relationship in cultured populations of rainbow trout (Islam et al. 1973). They would also concur with data available on other vertebrates, such as the barn owl, where this association occurs in relation to the size of dark spots (Roulin et al. 2001). However, in contrast to Islam et al. (1973), we observed a positive relationship between the numbers of dark spots below the lateral line and spawning time, i.e., females with higher numbers of dark spots tend to spawn later in the reproductive season than females with lower numbers of spots. Although, based on the present data, it is difficult to clarify the origin of this discordance between both data sets, it is possible that strain differences, management conditions, selection or genetic factors may explain, in part, these results. This is not unlikely, given that Islam et al. (1973) studied cultured strains in Japan, which that are usually subject to intense management in fish farms and, in contrast to a free-living population, are reared under controlled environmental conditions. These conditions are very different to those experienced by the naturalized populations of rainbow trout studied in this work, given that they inhabit a lake in southern Chile (Calafquén Lake), with particular limnic conditions related to special water characteristics (Campos 1984, Arismendi et al. 2011). In the case of selection and genetic factors, their effect on spawning time and spottiness would be expected to produce considerable variation among strains. This is possible given that, in rainbow trout, spawning time (Siitonnen and Gall 1989, Su et al. 1999) and spotting level (Kause et al. 2003) characters have a significant genetic determination, and therefore, may respond to selection pressure, which would alter their distribution in populations. In fact, Kause et al. (2003) have demonstrated that through artificial selection of the spottiness level of a cultured rainbow trout strain, it is possible to respond rapidly to selection pressure after a few generations.
Further studies on spotting variation in other naturalized populations of rainbow trout from Chile will be important to support the relationship of this parameter with biometric and reproductive parameters reported in this work. The analysis of the spotting variation in these populations, for example, according to the habitat characteristics, it will also be useful to add more data to clarify this issue.
CONCLUSION
No significant differences were observed in the mean number of dark spots below the lateral line between males and females in both reproductive seasons. This result suggests that spottiness below the lateral line is not sex-specific. Stepwise multiple regression analyses revealed that the body weight of males from year 2007 has a significant negative influence on the number of dark spots below the lateral line, while the reproductive variables egg diameter and spawning time of females from years 2007 and 2012, respectively, also have a relevant positive influence on this variable response. Overall, these results suggest that some biometric and reproductive variables may influence the skin spot variation of rainbow trout broodstocks living in natural environments.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
The transitory capture of the wild broodstocks of rainbow trout from Calafquén Lake, were authorized by the Chilean Undersecretary of Fisheries and Aquaculture (permit #2750-20-09-07). During the period of captivity, their reproductive management and subsequent release to the lake was carried out in accordance with the recommendations of the Guidelines for the Use of Fishes in Research (http://fisheries.org/guide-for-the-use-of-fishes-in-research).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The suggestions and comments of all those who helped to improve the final version of this manuscript, are gratefully acknowledged. We are also indebted to Chiguaico fish farm for the hatchery facilities and Lican Ray Fish and Game Club for its assistance in the field work during collection of fish. This study was partially financed by Subsecretaría de Pesca y Acuicultura, Ministerio de Economía, Fomento y Turismo, Gobierno de Chile, Project 2012-34-FAP-5. This work is dedicated to the memory of Alejandro Koffmann O’Reilly, an outstanding promoter of the rainbow trout restocking program of Lake Caláfquen.
REFERENCES
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]


