All published articles of this journal are available on ScienceDirect.
Leaf Litter Decomposition Rate in the Karst Ecosystem of the Community Forest in Wonodadi Village, Pracimantoro, Wonogiri, Indonesia
Abstract
Introduction
The availability of mineral nutrients in the karst ecosystem is specifically influenced by vegetation, which depends on how much nutrition is contributed by the litter of the stand through the decomposition process. The litter decomposition rate can be observed through physical changes and a decrease in litter weight. The community in Pracimantoro utilizes and maintains land by building community forests to optimize land in the karst area. This study aims to determine the litter decomposition rate in several community forest planting patterns in Pracimantoro.
Methods
Data were collected using the litter bag method by making sample plots measuring 0.04 ha and selected by purposive sampling, and placed diagonally. The rate of decomposition is described by the constant value (k).
Results
The karst community forest in Pracimantoro was dominated by Mahogany (Swietenia sp.), Acacia (Acacia auriculiformis), and Teak (Tectona grandis) stands. The rate of decomposition in the Pracimantoro karst community forest was 0.0268.
Discussion
Leaf litter decomposition over two months resulted in a decrease in litter weight of 33.05%. The duration of nutrient return in karst community forests can be estimated using the half-life approach.
Conclusion
The rate of litter decomposition is influenced by environmental conditions and litter quality. Environmental conditions that are suitable over a long period have a significant impact on the rate of litter decomposition.
1. INTRODUCTION
The very low carrying capacity of the karst area makes it prone to dissolution because it is vulnerable to climate change. Indonesia has a karst area of 15.4 million hectares, or 20% of the total area of Indonesia (Apriliana & Budiyanto, 2019). The rocks that make up the karst area contain carbonate rocks, making them easily damaged, especially when they come into contact with water with an acidic pH. Karst is a unique ecosystem because of the hydrological cycle below the surface of the soil, which means that only certain vegetation with low water requirements can grow in the karst area (Prabudimas and Budiyanto, 2020). This is because surface water flows into the holes in the cave system to form underground rivers (Faida et al., 2018). The underground water flow in the karst area in Indonesia, particularly in Pracimantoro, had a water discharge of 7,900 liters/second with 76 springs in 1998–1999 (Samodra, 2005).
The development of community forests and limited agriculture in the karst of Pracimantoro District is carried out as a form of area preservation. Hudiyani et al. (2017) stated that forests are one of the pillars of community life, maintain ecosystem balance, and provide a means for social interaction. According to Salim, & Budiadi, (2014), the level of plant diversity in an area affects soil fertility. The circulation of nutrients in tropical forest areas occurs specifically where vegetation functions as a provider of nutrient reserves. Most of the nutrients in the forest are stored in forest stands, and some are in the soil layer (Siagian et al., 2021). The amount of nutrient input to an area is influenced by litter from the vegetation above it through the decomposition process (Tongkaemkaew et al., 2018).
Forest productivity depends on the amount of litter that falls, and dense forest canopies tend to contribute more litter than sparse canopies (Kusmana and Yentiana, 2021). Nursal et al. (2015) reported that dense forest vegetation can influence the decomposition process of litter on the forest floor. A closed canopy affects the entry of light to the forest floor. Less light entering the forest floor creates more humid conditions, favoring decomposers (Wibowo & Rizqiyah, 2014). Furthermore, Wibowo & Rizqiyah (2014) stated that the increase in decomposers is directly proportional to the speed of litter decomposition.
Vegetation as a litter producer plays an important role in the forest nutrient cycle, maintaining and restoring nutrients and soil fertility. Litter that falls on the forest floor becomes a habitat for microorganisms and fungi, facilitating the decomposition process. The ability to decompose litter is influenced by environmental factors and litter chemistry (Aerts, 1997). The karst landscape is an interesting research location due to its unique ecosystem. Research on karst areas is still rare in Indonesia, including studies measuring the rate of litter decomposition. Pracimantoro District has potential community forests that have never been studied regarding litter decomposition rates. Variation in vegetation structure, species composition, and planting patterns in community forests is believed to influence microclimate conditions and the presence of decomposing microorganisms. Therefore, this research is essential to obtain information on litter decomposition rates to maintain the stability of mineral nutrients in the karst ecosystem and support the sustainability of the karst area.
2. MATERIALS AND METHODS
2.1. Site Study and Research Design
The study was conducted in the Pracimantoro karst ecosystem community forest, Wonogiri Regency. The study was carried out in community forests with three different types of land management: monoculture, polyculture, and agroforestry. This study aims to determine the rate of decomposition in the three community forest planting patterns.
This study is an analytical study conducted with a quantitative approach, focusing on comparing the percentage of litter mass loss from each land area, with environmental parameters used as control variables. The study was conducted observationally by placing litter bags on each land-use pattern and observing them for a specified period without any manipulation.
Measurement plots were determined by purposive sampling, considering the representativeness of land management patterns (Tongkaemkaew et al., 2018). Field data collection from each type of community forest land management was carried out by creating plots with a 3-case × 3 system × 3-repetitions design, resulting in a total of 9 measurement plots (Tongkaemkaew et al., 2018). The data collection plots were square with an area of 0.04 ha, as commonly used in karst areas (Salim & Budiadi, 2014; Moro et al., 2016; Tongkaemkaew et al., 2018). Furthermore, three points intersecting the diagonal of each measurement plot were selected as locations for placing research samples, with 12 litter bags placed in each plot (Tongkaemkaew et al., 2018).
2.2. Literature Review Strategy
A literature review was conducted to support the interpretation of field data and to contextualize the decomposition rate of leaf litter in karst ecosystems. The literature search was carried out using several academic databases, including ScienceDirect, Scopus, Google Scholar, and DOAJ, between January and April 2024.
This study involved the collection of leaf litter from cultivated tree species commonly found in community forests (e.g., Swietenia mahagoni, Tectona grandis, and Acacia auriculiformis). All plant materials were collected non-destructively from the forest floor and did not involve endangered or protected species as listed by the IUCN Red List or CITES. The research complied with all relevant institutional, national, and international guidelines on plant research. Fieldwork was conducted with the permission of local authorities and with the cooperation of forest farmer groups in Wonodadi Village, Pracimantoro, Wonogiri, Indonesia.
2.3. Data Collection
2.3.1. Litter decomposition
The measurement of the litter decomposition rate was carried out using the litter bag method. Data were collected with a litter bag weighing 20 grams/bag by arranging the litter, especially on large leaves, so that layering does not occur, thus slowing down microbial respiration in decomposition (Berg & McClaugherty, 2008; Devianti & Tjahjaningrum, 2017). And then, a litter bag was placed on the forest floor (Devianti & Tjahjaningrum, 2017; Susanto et al., 2021) and taken every two weeks (Salim & Budiadi, 2014; Karina et al., 2022). Furthermore, the litter will be dried using an oven at a temperature of 105 °C for 24 hours until the weight is constant (Kusmana and Yentiana, 2021), then the weight reduction is calculated. The analysis of the decomposition rate uses the Olson (1963) equation as shown in Eq. (1) (Aerts, 1997; Kusmana & Yentiana, 2021). Data analysis was carried out using SPSS version 16.
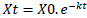
Notes: k = litter decomposition rate; T = observation time (weeks)
2.3.2. Environmental Parameters
Environmental parameter measurements were conducted every 15 days in the morning (07:00 - 09:00 WIB), afternoon (12:00 - 14:00 WIB), and evening (16:00 - 18:00 WIB) (Annisa et al., 2015) along with the day the samples were taken. Measurement of temperature and humidity using a thermohygrometer. The results obtained were then presented as a daily relative average table. Data analysis was calculated using Eq. (2) (Setyowati, 2008; Annisa et al., 2015; Simbolon et al., 2022).
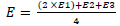
Notes: E = environmental parameters; E1 = morning measurement; E2 = afternoon measurement; E3 = evening measurement
3. RESULT AND DISCUSSION
3.1. Vegetation of the Karst Community Forest
The results of vegetation research in the Pracimantoro karst community forest are presented in Table 1. Pracimantoro District is a karst mountain area whose main structure is limestone and carbonate rock. The development of community forests in karst areas was chosen to rehabilitate land and preserve the hydrological system (Nasrudin & Parikesit, 2020). Community forest management is carried out traditionally by implementing three forms of planting patterns, namely monoculture (Fig. 1a), polyculture (Fig. 1b), and agroforestry community forests (Fig. 1c). In addition to forestry plants, several species of understory plants were also found as medicinal plants and crops, especially in agroforestry patterns. The medicinal plants found were turmeric (Curcuma longa), lempuyang (Zingiber zerumbet), and ginger (Zingiber officinale), while taro (Colocasia esculenta) is a crop.
| Community Forest Planting Patterns | Tree Species | Other Components |
|---|---|---|
| Agroforestry | Teak, Sengon, Acacia, Sandalwood, Mahogany, Melinjo, Petai, Rosewood |
Livestock: Cows, Bees Agriculture: Ginger, Turmeric, Taro, Lempuyang |
| Polyculture | Acacia, Mahogany, Teak | - |
| Monoculture | Mahogany, Acacia, Bintaro | - |
The results of species identification in the three forms of community forest management (Fig. 1 and Table 1) are divided into six families: Fabaceae, Meliaceae, Lamiaceae, Gnetaceae, Santalaceae, and Apocynaceae. According to Prabowo et al. (2024), the stands originating from the Fabaceae and Meliaceae families were the dominant ones. Previously, Faida et al. (2018) also reported that the karst ecosystem is composed of vegetation with long and deep roots, such as Tectona grandis, Swietenia sp., Acacia auriculiformis, Paraserianthes falcataria, and Melaleuca cajuputi. As shown in Table 2, Mahogany (Swietenia mahagoni), Teak (Tectona grandis), and Acacia (Acacia auriculiformis) were the most dominant species and had the highest IVI in the Pracimantoro karst community forest. In accordance with research conducted by Adiputra et al. (2024), Mahogany (Swietenia mahagoni) dominates the forest in the Gunung Sewu Karst area, followed by Teak (Tectona grandis). These species are trees with commercial purposes as sawmill timber (Table 2) (Gurashi et al., 2024).

Community forest management in Pracimantoro District based on planting patterns. a) monoculture, b) polyculture, and c) agroforestry.
They are selected in community forest management because they have high economic value for the community (Hudiyani et al., 2017). In addition, from an ecological perspective, these species have roots that can penetrate deep into the soil and possess high adaptive abilities to the climate in the karst area (Samodra, 2005). Mahogany stands have the highest IVI of 105.34% (Table 2). Berg & McClaugherty (2008) conveyed that species with the highest IVI have a greater chance in an ecosystem community to maintain their growth. In addition, there are species of stands that have IVI values that are not much different, namely acacia and teak species with values of 71.49% and 71.38%, respectively. The magnitude of the IVI values of the two species means that both have positions, roles, and growth abilities that are not much different in their communities (Adiputra et al, 2024). These results show that these species have a higher position than other species found, especially their ability to adapt to the environment as indicated by the magnitude of the IVI value (Pertiwi & Chairul, 2024).
Vegetation plays an important role in the formation of community forests. Based on Table 2, it can be concluded that the community forest in Wonodadi Village has a dense forest with a stand density of 5,275 ind/ha. Furthermore, the highest density is given by mahogany, teak, and acacia stands (Table 2). High density illustrates that the species has the largest number of other species at that location (Zulkarnain, 2015). This is then in accordance with the condition of the vegetation planted, without taking into account the planting distance and without carrying out thinning activities.
3.2. Enviromental Parameters
3.2.1. Temperature
The environmental parameters at the study site are presented in Table 3. The average daily air temperature in the Pracimantoro karst community forest is 30.5°C. This value is slightly higher than the average air temperature in karst ecosystem forests in China, which was 30.3°C (Zhu et al., 2021). Based on the classification index by Setyowati (2008), the average air temperature in the Pracimantoro karst community forest indicates that the area has a hot microclimate.
Community forests with a monoculture pattern have the highest average daily temperature of 31.65°C. The high temperature is due to the species planted on monoculture land having light canopies. The thin canopy allows high light intensity to penetrate to the forest floor, resulting in low air humidity under the stand (Siagian et al., 2021). Indirectly, air temperature significantly affects the reduction of litter mass because it also influences microbial activity (Devianti & Tjahjaningrum, 2017).
3.2.2. Relative humidity
Based on Table 3, the average relative humidity in the karst community forest was 62.55%, with the highest average of 64.82% in agroforestry land. Susanti & Halwany (2017) stated that air temperature in an area is inversely proportional to its air humidity. This is consistent with the results of this study, showing that community forests with higher humidity have relatively lower air temperatures. The classification of climate conditions based on air humidity by Setyowati (2008) indicates that the Pracimantoro karst community forest has a dry climate, as it has a relative humidity of <70%.
Air humidity is a critical environmental factor in the litter decomposition process because it affects the presence of decomposing microorganisms. Appropriate humidity (>70%) can accelerate litter decomposition even in areas with high air temperature (Devianti & Tjahjaningrum, 2017). Jupri et al. (2024) stated that air humidity in an area is influenced by air temperature, light intensity, air pressure, water availability, and vegetation. Agroforestry community forests have vegetation with medium to dense canopies, creating higher air humidity. However, the karst community forest, with air humidity below 70%, experiences a slower rate of decomposition.
| No | Species | Family | K (n/ha) | KR (%) | FR (%) | DR (%) | IVI (%) |
|---|---|---|---|---|---|---|---|
| 1 | Swietenia mahagoni | Meliaceae | 2150 | 23.70 | 24.00 | 40.58 | 105.34 |
| 2 | Acacia auriculiformis | Fabaceae | 1125 | 40.76 | 24.00 | 26.16 | 71.49 |
| 3 | Tectona grandis | Lamiaceae | 1250 | 21.33 | 24.00 | 23.68 | 71.38 |
| 4 | Cerbera manghas | Apocynaceae | 400 | 7.58 | 4.00 | 3.03 | 14.61 |
| 5 | Paraseriantes falcataria | Fabaceae | 125 | 1.90 | 4.00 | 2.24 | 8.61 |
| 6 | Gnetum gnemon | Gnetaceae | 100 | 2.37 | 4.00 | 1.51 | 7.40 |
| 7 | Parkia speciosa | Fabaceae | 25 | 0.47 | 4.00 | 1.23 | 5.70 |
| 8 | Santalum album | Santalaceae | 50 | 0.95 | 4.00 | 0.38 | 5.33 |
| 9 | Swietenia macrophylla | Meliaceae | 25 | 0.47 | 4.00 | 0.79 | 5.27 |
| 10 | Dalbergia latifolia | Fabaceae | 25 | 0.47 | 4.00 | 0.38 | 4.86 |
| Total | 5275 | 100 | 100 | 100 | 300 | ||
| Community Forest Planting Patterns | Temperature (oC) | Relative humidity (%) | Soil pH | Light Intensity (%) |
|---|---|---|---|---|
| Agroforestry | 29.96 | 64.83 | 7,3 – 7,8 | 78.11 |
| Polyculture | 29.88 | 63.98 | 7,5 – 8 | 87.55 |
| Monoculture | 31.65 | 58.87 | 7 -7,8 | 83.53 |
| Average | 30.50 | 62.55 | 7 - 8 | 83.06 |
3.2.3. Soil pH
Based on the results of field analysis, the soil pH in the Pracimantoro karst community forest ranged from 7.0 to 8.0. The research by Prabowo et al. (2024) also showed that the soil pH in the Pracimantoro karst community forest ranged from 7.56 to 8.03. This indicates that the soil in the karst community forest is neutral to slightly alkaline. The karst structure, consisting of limestone (CaCO3), results in high base cations, making the soil alkaline. Muhsin et al. (2017) stated that the optimal soil pH for litter decomposition is 6.5 to 7.0. Furthermore, Nursal et al. (2015) added that alkaline soil can interfere with the activity of decomposing microorganisms, causing the decomposition process to proceed slowly. In their research, Susanto et al. (2021) concluded that soil pH has a 50% influence on the litter decomposition process when combined with appropriate humidity.
3.2.4. Rainfall
Rainfall data obtained from the Central Statistics Agency of Wonogiri Regency over a period of 10 years (2014–2023) show that Wonogiri Regency has rainfall ranging from 1,108 to 3,066mm/year, with an average of 2,084.1mm/year. According to the classification by Schmidt and Ferguson, Wonogiri Regency falls under climate type C (rather wet), with a Q value of 55.89%.
Based on data from the Ministry of Public Works and Spatial Planning, Directorate General of Water Resources, BBWS Bengawan Solo (2024), rainfall in Wonogiri Regency averaged 134.43mm, and during observations from May to July, it averaged 25.89mm. According to the Meteorology, Climatology, and Geophysics Agency (BMKG), the average rainfall in that year indicates that Wonogiri Regency experiences medium rainfall, while the average monthly rainfall during that period is classified as low. Devianti & Tjahjaningrum (2017) stated that rainfall affects humidity and environmental temperature, and high rainfall can facilitate litter breakdown, accelerating decomposition. The low rainfall during the research period made temperature and humidity less supportive of the decomposition process.
3.2.5. Light Intensity
The light intensity in the three types of planting patterns in the community forest ranged from 78.11% to 87.55%, with an average of 83.06% (Table 3). Light intensity in different types of community forests is influenced by canopy density. Siagian et al. (2021) stated that a dense canopy creates shade, preventing sunlight from reaching the forest floor. The light intensity produced by solar radiation can also accelerate the photodegradation of recalcitrant chemical compounds (Huang et al., 2024).
Community forests with an agroforestry planting pattern have the lowest light intensity. The diverse strata and wide canopy of the constituent stands are influencing factors (Siagian et al., 2021). In contrast, monoculture land has the highest light intensity because the planting pattern includes Acacia and Bintaro, both of which have light canopies. The temperature and humidity of the air beneath the stand are also impacted by light intensity (Nurjanto et al., 2016). Denser stands block more sunlight from reaching the forest floor, resulting in lower light reception (Simbolon et al., 2022), which in turn increases humidity levels.
3.3. Litter Decomposition Rate
3.3.1. Litter Weight Reduction
The process of leaf litter decomposition can be seen directly by observing the color changes of the leaves along with changes in their physical structure (Fig. 2). The initial stage of litter decomposition is the occurrence of physical changes in the litter, which will become more intensive over time (Karina et al., 2022). Color changes in leaf litter can occur due to the breakdown of the green leaf substance (chlorophyll) in the leaves, which were originally green, turning yellow, brown, and changing to other darker colors (Berg & McClaugherty, 2008). Physical changes in leaf litter during observation did not show any striking changes (Fig. 2). However, the structure of the litter began to become brittle and break and crack. Changes in litter structure are presented in Fig. (2a) (monoculture), Fig. (2b) (polyculture), and Fig. (2c) (agroforestry). Damage to the leaf litter structure on monoculture and agroforestry is more visible when compared to damage to the leaf litter structure on polyculture land. Siagian et al. (2021) stated that the decomposition process will change the structure of leaf litter to become more brittle and then form flakes.

Physical changes of litter in karst community forests for 60 days.
(a) Monoculture; (b) Polyculture; (c) Agroforestry.
Dominantly, the litter decomposition process is accompanied by a decrease in the dry weight of the litter. The diverse composition of the stand causes variation in litter weight loss rates in the field. Litter weight loss occurs fluctuatingly depending on supporting factors over time (Nursal et al., 2015). The decrease in litter weight in the Pracimantoro karst community forest is presented in Fig. (3).
The average final litter weight, based on Fig. (3), in agroforestry, polyculture, and monoculture community forests was 13.04 g, 14.23 g, and 12.90 g, respectively. The most significant decrease in litter weight was observed in monoculture community forests, at 35.50%. This value is slightly higher than the weight loss in agroforestry community forests, which reached only 34.78% of the initial weight. The magnitude of litter weight loss can be influenced by various factors, including environmental and climatic conditions, as well as the quality of the litter from the standing species.
The results shown in Fig. (4) indicate that the average percentage of dry litter weight loss in all types of community forests over 2 months was 33.05%. The decrease in dry litter weight increased over time. The percentage decrease in litter weight observed in this study is smaller than that reported by Rumambi et al. (2018), who found a weight loss of 32.37% during one month of decomposition, but larger than that reported by Karina et al. (2022), who observed a 10.4% decrease over 3 months.
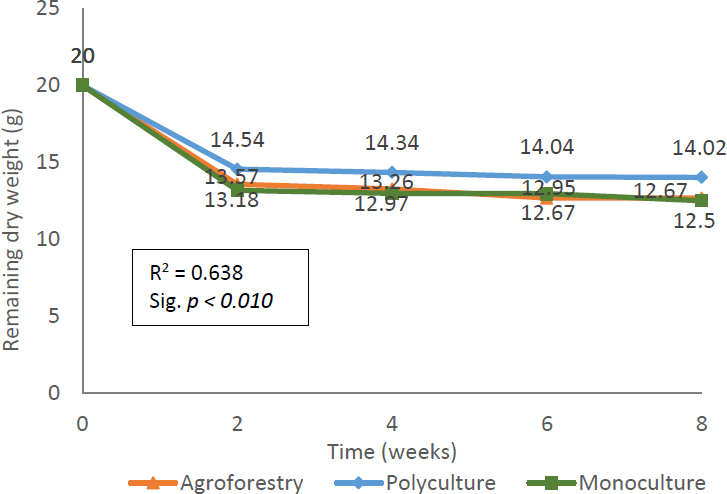
Graph of litter weight reduction in Pracimantoro Karst Community Forest.
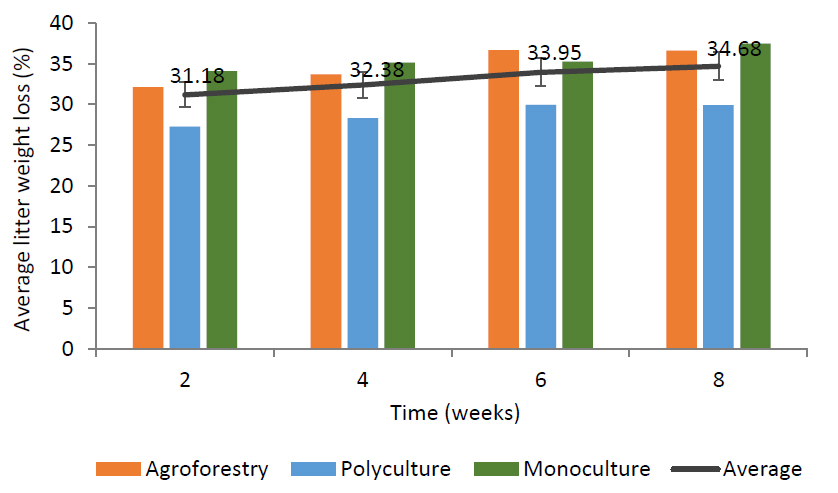
Average percentage of litter weight reduction in Pracimantoro Karst community forest.
| Species | Chemical Content (%) | Sources | ||||
|---|---|---|---|---|---|---|
| P | N | C | Lignin | Polyphenols | ||
| Tectona grandis | 0.08 | 0.93 | 48.7 | 15.18 | 11.85 | Umayya, 2017 |
| Swietenia sp. | 0.48 | 0.82 | 18.1 | 16.6 | 25.26 | Umayya, 2017 |
| Acacia auriculiformis | 0.29 | 3.9 | 49.6 | 19.6 | 7.7 | Oyun et al., 2006 |
Polyculture community forests with environmental conditions that are almost similar to agroforestry forests tend to experience the slowest decrease in litter weight (Fig. 4). The decrease in litter weight is only 28.85% of its initial weight (Fig. 3). This is due to the dominance of stands with low litter quality so that they are difficult to decompose. These stands include: mahogany, teak, and acacia. Susanto et al. (2021) stated that the loss of litter weight is caused by differences in litter chemistry.
Based on Table 4, the three dominant species in the study site have high lignin and phenol chemical content, so they have low litter quality and are difficult to decompose. Salim & Budiadi (2014) emphasized that a land will experience a slow decomposition process if it is dominated by stands that have high lignin and polyphenol content in their litter. According to Hairiah et al. (2006), high-quality litter is litter with a C/N ratio of <25%, lignin <15%, and polyphenol content <3% which will be easily decomposed. Lignin and polyphenol content are difficult to decompose because of their resistance to decomposer bacteria (Darmayanti & Ridesti 2012). Aerts (1997) stated that the lignin content of evergreen stands is relatively high, so that it can control the speed or slowness of the litter decomposition process. Mahogany and Acacia have higher lignin than teak because both are included in evergreen stands, while teak is not (Table 4).
In high concentrations, lignin and polyphenol content can inhibit the rate of decomposition, whereas the N and P content in the litter can accelerate the rate of decomposition (Aerts, 1997; Huang et al., 2024). Therefore, the litter weight loss in monoculture community forests was highest because mahogany and acacia, as their constituent species, have higher N and P content than teak, which is not their constituent species (Table 4). Furthermore, agroforestry community forests have the second-highest weight loss below monoculture forests because, in the agroforestry land, the three stands above are not the primary constituent stands but are combined with other stands.
3.3.2. Litter Decomposition Rate
The rate of leaf litter decomposition in several forms of community forest land management is presented in Fig. (5). The litter decomposition rate was illustrated by the decomposition constant (k). The results of the analysis show that the average decomposition rate is 0.0268. The highest decomposition rate is on land with a monoculture planting pattern of 0.0293, then in an agroforestry planting pattern of 0.0277, and the smallest decomposition rate of 0.0229 in community forests with a polyculture planting pattern (Fig. 5). The decomposition rate is influenced by environmental conditions such as vegetation, temperature, air humidity, and rainfall. [16] Vegetation density and the amount of rainfall are the main factors causing high or low temperatures and air humidity in an area.
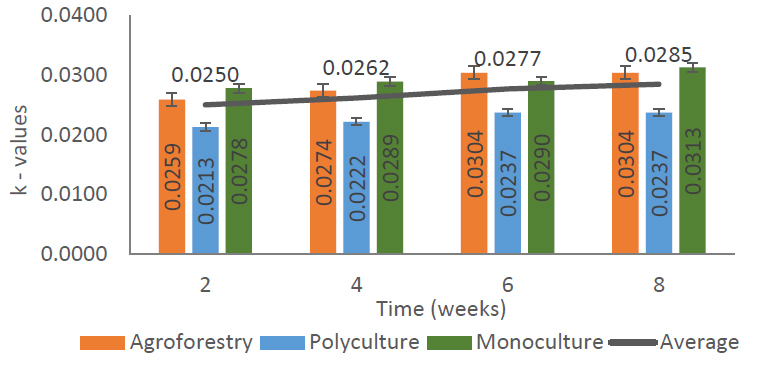
The litter decomposition rate (k) in several planting patterns of community forest management in the Pracimantoro Karst ecosystem. The X-axis represents the type of planting pattern, while the Y-axis shows the decomposition rate constant (k) as calculated from dry mass loss.
The decomposition rate was calculated from the weight loss that occurred during the research process. The litter decomposition process occurs in two stages, namely the reduction in water content of the litter along with dissolved chemicals in water, such as glucose and phenol compounds, and further decomposition with the breakdown of complex litter chemicals such as lignin and cellulose (Nursal et al., 2015).
Based on the measurements carried out, Wonodadi Village has low rainfall, a dry and hot climate, so the decomposition process is slow. It was recorded that the Pracimantoro karst community forest had a very low k value when compared to research at the Nglanggeran Gunungkidul Ancient Volcano by Moro et al. (2016), which had a positive k value ≤3 as a weathering constant. However, the results of the measurement of the litter decomposition rate are higher than the research of Karina et al. (2022) at KHDTK, Bengkulu University, whose highest decomposition constant value was <0.02. Even so, the decomposition rate that occurs in the Pracimantoro karst community forest is difficult to observe because it has a very small k value (<0.1). Moro et al. (2016) stated that the decomposition constant value (k) <0.1 will be difficult to observe, especially during the dry season.
Based on Fig. (5), the decomposition rate that occurs in the karst community forest has a number that is not much different from one another. The k value tends to increase in all forms of land management as the observation time increases. Devianti & Tjahjaningrum (2017) stated that the suppression effect of litter decomposition will occur since the litter shrinks by 30 - 40% of the initial weight of organic material. Microclimate conditions in an area will affect the presence of decomposing microorganisms and affect their speed in decomposing litter (Karina et al., 2022).
An environment that is always wet and humid will accelerate the decomposition process of litter on the forest floor. The hot and dry environmental conditions in the Pracimantoro karst forest have an impact on the slow rate of decomposition in the area. Berg & McClaugherty (2008) said that the value of the litter decomposition rate can be interpreted as the rate of return of nutrients in an ecosystem. The rate of return of soil nutrients depends on the rate of litter destruction and the decrease in dry weight of litter over a certain period of time.
3.4. Model of the Relationship between dry Litter Weight and Time
The relationship model of dry weight of the remaining litter with time can be described using an exponential regression model (Fig. 6). The rate of litter decomposition is a dynamic process. Litter decomposition has different dimensions over time because it is highly dependent on factors that influence the rate of litter decomposition (Devianti & Tjahjaningrum, 2017). The longer the decomposition process takes place, the litter will continue to experience a decrease in litter weight until the litter reaches the final stage of composting.
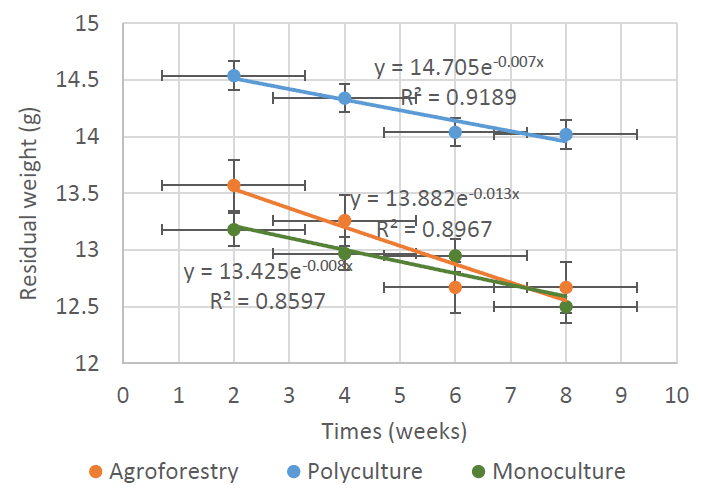
Exponential regression model of the relationship between remaining litter dry weight and decomposition time (weeks). The X-axis represents the duration of decomposition (weeks), while the Y-axis shows the remaining litter mass (grams). The curve illustrates the exponential decay trend of litter mass over time.
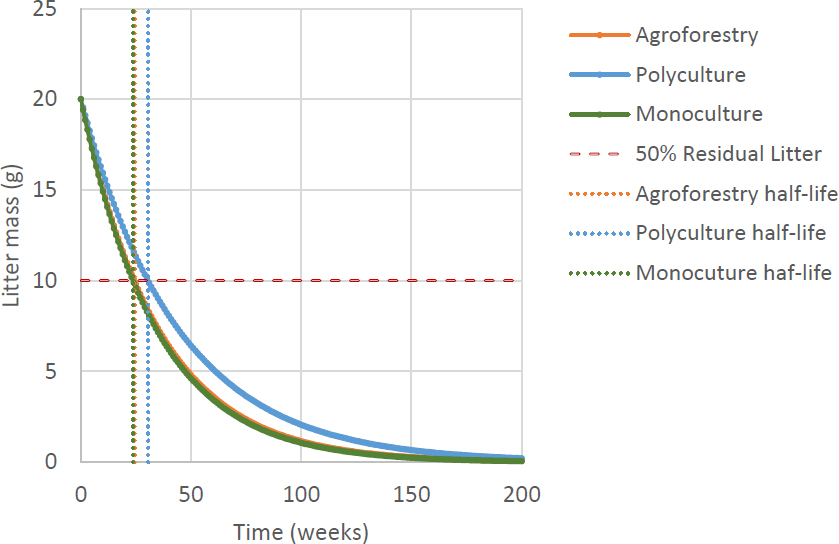
Estimation of leaf litter weight in several types of community forest management in the Pracimantoro Karst ecosystem based on Olson’s exponential regression model. The X-axis represents time (weeks), and the Y-axis represents the estimated remaining dry litter weight (grams). Shorter half-life values indicate faster decomposition rates.
Based on the exponential equation created, the decomposition process in the litter in the Pracimantoro karst community forest cannot be predicted when it will reach zero. However, the reduction in the weight of the remaining litter can be estimated using half-life calculations. Siagian et al. (2021) said that an R-square value of more than 0.5 indicates a good relationship between the two variables. The decay of the remaining weight of the litter is suitable to be presented in an exponential graph because the initial process of weight loss will be greater and slow down over time (Mahmudi et al., 2011). Nurjanto et al. (2016) stated that the magnitude of the decrease in litter weight is never constant but always changes over time. From the equations obtained from the three forms of community forest management, it can be estimated that the cycle of decreasing the remaining weight in the litter decomposition process from monoculture forests, polyculture forests, and agroforestry sequentially occurs every 23.64 weeks, 30.52 weeks, and 24.31 weeks (Fig. 7). The smaller the half-life value, the faster the decomposition process will take place. Zhu et al. (2021) stated that the average litter in tropical forests throughout the world will decompose for 0.50 - 3.03 years. However, leaf litter experienced variations in decomposition time between 1 - 5 years (Prescott, 2010).
The length of the litter decomposition process in the Pracimantoro karst community forest shows varying results, with a notable difference among the three community forest land management types. Based on its half-life, leaf litter in agroforestry and monoculture lands has a similar decomposition time. The rate of litter decomposition is influenced by the type of stand, environmental conditions, and litter quality (Aerts, 1997; Prescott, 2010; Huang et al., 2024). Environmental conditions that are suitable continuously and over a long period have a significant impact on the rate of litter decomposition (Prescott, 2010; Rumambi et al., 2018). However, Prescott (2010) emphasized the importance of including a moisture threshold in Olson’s decomposition model, considering that karst areas are dry and experience water limitations. This modification provides a more realistic description of decomposition dynamics in dry karst areas.
4. STUDY LIMITATIONS
This study has several limitations. First, the limited observation time may not reflect the entire litter decomposition cycle, which can last for 1–5 years depending on the type of litter and microclimate conditions. Second, the dry season during data collection caused high temperatures and low humidity, which may slow down the activity of microorganisms and affect the accuracy of the decomposition rate estimation. Third, this study only observed macro-environmental variables (temperature, humidity, pH, and light intensity) and did not measure the diversity of soil microbes or fauna, which also play an important role in the decomposition process. In the future, further research should be conducted during the rainy season and involve further biological parameters and chemical tests of litter and soil. In addition, considering that the monoculture pattern has the fastest estimated decomposition time compared to other planting patterns, the suggestion for further research is to conduct a study on the influence of seasons (multipool) and interactions between litter in polyculture and agroforestry planting patterns on the rate of litter decomposition.
CONCLUSION
The average decrease in litter weight across all forms of karst community forest management was 33.05%. Monoculture forests had the highest decrease in litter weight at 35.50%, followed by agroforestry forests at 34.78%, and polyculture forests at 28.82%. The dominance of Mahogany (Swietenia sp.), Acacia (Acacia auriculiformis), and Teak (Tectona grandis) as constituent species greatly influenced the rate of litter decomposition, as these three species have high lignin and polyphenol content. The average rate of leaf litter decomposition in karst community forests was 0.0268. The half-life of litter decay in monoculture, polyculture, and agroforestry lands was 23.64 weeks, 30.52 weeks, and 24.31 weeks, respectively.
AUTHOR’S CONTRIBUTIONS
The authors confirm contribution to the paper as follows: S.R.R., N.M.: Conception and design; S.R.R.: Data collection; S.R.R., N. M., S.: Analysis and interpretation of results; Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| IVI | = Important Value Index |
| P | = Phosphorus |
| N | = Nitrogen |
| C | = Carbon |
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Repository Universitas Brawijaya, Indonesia at https://repository.ub.ac.id/id/eprint/132018/, reference number 36”. (Umayya, L (2017) Effect of litter type to earthworm Pontoscolex corethrurus development. Undergraduate Universitas Brawijaya, Malang, Indonesia).
FUNDING
This research was funded by non-state budget (non-APBN) support from Universitas Sebelas, Indonesia Maret under Contract Number 194.2/UN27.22/PT.01.03/2024. The authors would like to express their gratitude to LPPM Universitas Sebelas Maret (UNS) for the support provided through a research grant with contract number 371/UN27.22/PT.01.03/2025.
ACKNOWLEDGEMENTS
Thanks to the Pracimantoro District Government for granting permission to conduct this research and to the Extension Officer of the Forestry Service Branch Office Region XI for assisting with the research. Thanks also to all members of the forest farmer groups in Wonodadi, who were cooperative and facilitated research activities in the field, allowing the necessary data to be collected. The authors would like to express their gratitude to LPPM Universitas Sebelas Maret (UNS) for the support provided through a research.


