All published articles of this journal are available on ScienceDirect.
Assessment of NORM in Mining Residues and Environmental Materials from Sefwi Awaso Bauxite Mine, Ghana
Abstract
Background
A surge in illegitimate mining is threatening the legitimacy of mining industries such as bauxite mining in Awaso. An awareness of radioactivity problems is essential when evaluating any application. Public perceptions and apprehensions regarding risks associated with mining activities must be acknowledged.
Objective
The primary aim is to evaluate the radioactivity in water and soil from the Sefwi Awaso bauxite mine and its surroundings, as well as to determine the health risks to mine workers and the local population.
Methods
Mining residues from the bauxite mine and surrounding area were analyzed for radioactive concentrations using direct gamma-ray spectrometry with a high purity germanium detector. Genie-2000 program was used for qualitative analysis of radionuclide.
Results
Soil, bauxite ore, and red mud all had different concentrations of 226Ra, 232Th, and 40K based on their respective activities, thus: (29 ± 3.2) Bq kg-1, (32 ± 4) Bq kg-1, and (179 ± 18) Bq kg-1; (39 ± 4) Bq kg-1, (97 ± 11) Bq kg-1, and (15 ± 2) Bq kg-1; (45 ± 5) Bq kg-1, (64 ± 7) Bq kg-1, and (125 ± 19) Bq kg-1. In the community water samples, the average activity concentrations of 226Ra, 232Th, and 40K were (1.0 ± 0.9) Bq l-1, (3.2 ± 0.6) Bq l-1, and (16 ± 5) Bq l-1, respectively, which are all well within the global average activity concentrations. The committed effective dose was 0.63 ± 0.08 mSv, and annual effective dose estimates for the mine and community were (75 ± 5) µSv and (26 ± 3) µSv, respectively.
Conclusion
The bauxite mining operation does not significantly increase activity concentrations, suggesting an annual dose increase well below the 1 mSv y-1 limit for public exposure.
1. INTRODUCTION
Mining has been identified as one of the potential sources of exposure to Naturally Occurring Radioactive Materials (NORM), and they exist in various kinds of natural sources (UNSCEAR 2000). The preeminent concentration of these terrestrial radionuclides regularly originates in geological materials such as igneous rocks and ores. Human activities around such geological settings might lead to a higher concentration of radionuclides in the environment, thereby increasing human exposure to ionizing radiation. If these activities are not well managed or controlled, consequently, under adverse conditions, they may present a radiation risk to individuals. Mining and various industrial activities involving minerals and raw materials, including phosphate, monazitic sand, bauxite, coal, and metals, can elevate the effective dose received by workers and the public from natural sources such as soil, water, and air (El Zrelli R. et al. 2019, Hamed M.M. et al. 2016, Goronovski A. et al. 2021, Galhardi J.A. et al. 2017, Huang Y.J. et al. 2015 and El Hajj T.M. et al. 2019). Radionuclides may be unevenly distributed among the numerous components generated during the mining of minerals from the Earth's crust and their subsequent physical or chemical processing (Liu H. et al. 2012 and >van der Sloot H.A. et al. 2017).
Mining entails the extraction of ores along with the accompanying waste material, which includes non-mineralized or low-grade rocks (Michalik B. et al. 2018)>. Mined ores are further treated to separate precious minerals from gangue, resulting in concentrates and tailings. Mining waste and mineral processing tailings necessitate proper disposal and management due to their potential significant environmental impact (Goronovski A. et al. 2018). These materials may also comprise trace elements, including radionuclides.
The radioactive materials that occur naturally in the environment are thorium and uranium decay chains and potassium (Alashrah, S. and Taher, A. E. 2017). The emanation of radiation from soil and rock is due to both the decay of the parent radionuclides to their daughter radionuclides and also depends on the mineral composition of the Earth's crust. Many industries, particularly the mining industries, have operated for a long time without knowing that their operations could give rise to NORM in environmental materials as well as in mining residues (Brian, H. et. al. 2012 and >Darko, E. O. et. al. 2005).
Bauxite is considered radioactive primarily due to the presence of naturally occurring radionuclides, particularly 238U, 232Th, and 40K. These elements are concentrated in bauxite because the geological processes that form bauxite deposits also facilitate the accumulation of these radioactive materials. Significant activity concentrations of these radionuclides have been found in bauxite in a number of investigations that show increased levels in comparison to surrounding soils (Adams Udoji >Itodo et. al. 2023). The mineral makeup and unique geological circumstances of mining regions affect the concentration of these elements in bauxite, resulting in increased radiation levels that call for monitoring for any exposure-related health hazards (Aydan Altıkulaç 2022).
Radioactive substances are unique among all contaminants that adversely influence the quality of surface and groundwater and provide a dual hazard from toxicity and ionizing radiation. Many of these radionuclides have a large energy potential, which makes them both advantageous and dangerous. Radioactive materials are extremely valuable for energy and medicinal applications because of their special characteristics. Compounds and their byproducts can, however, be released into the environment through the mining, manufacture, use, and disposal of these materials, endangering both people and ecosystems (Kate Campbell 2021).
The ways that alpha, beta, and gamma radiation are exposed and how they affect tissue vary greatly. Exposure to ionizing radiation raises the risk of developing cancer and is mutagenic in all forms (WHO 2005 and EPA 2009). Although alpha particles have the lowest penetration depth, they are the most ionizing. The health implications, however, can be severe if an alpha-emitting source is consumed because alpha particles are the most harmful type of radiation. Although less harmful than alpha particles, beta particles can nonetheless damage cells and cause DNA mutations due to their greater penetration. Even when the source is not ingested orally, gamma radiation is very penetrating and can result in serious cell damage and mutagenesis.
The study undertaken was mainly to assess the NORM in water and soil from the mine and the environs of Sefwi Awaso Bauxite Mine and to estimate the health risk to both mine workers and community members residing in the vicinity of the mining area. Specific objectives were to identify and estimate the levels of 238U, 226Ra, 232Th, and 40K in bauxite mining and also to estimate the exposure of workers and the public due to the consumption of water from the area as a result of the mining activities.
2. MATERIALS AND METHODS
2.1. Description of the Study Area
Sefwi Awaso is a town located in the municipality called Bibiani-Anhwiaso-Bekwai in the Western North Region of Ghana with the geographical coordinates of 6°14'0”N and 2°16'0”W. It is at an elevation of 166 meters above sea level with a population of 29,748, according to the Ghana Population and Housing Census (Ghana Population and Housing Census 2010>). The habitats are predominantly peasant farmers, with a few engaging in artisanal mining.
2.2. Materials
2.2.1. Collection of Soil, Bauxite Ore, and Red Mud Samples
Soil, mining residue, and bauxite ore samples were collected within the perimeter of the Awaso bauxite mine and 5 km away from the surrounding communities. A simple random sampling technique was adopted where the samples were collected using a manual sampling tool, a shovel, into a polythene bag. The samples were aggregated from three different but close (within a meter radius) locations into one representative sample with a single coordinate. These coordinates were plotted as shown in Fig. (1). A total number of eight aggregated samples of all kinds, comprising soil, ore, and red mud samples, were collected at 5 cm depth using the hand shovel. Labeled polythene bags were used in the collection of the soil, ore, and red mud samples, respectively. The Awaso bauxite mine contains a plant that is mostly used for crushing. Thus, the boulders are crushed and washed on a machine, producing red mud, which is made from the collection of residues.
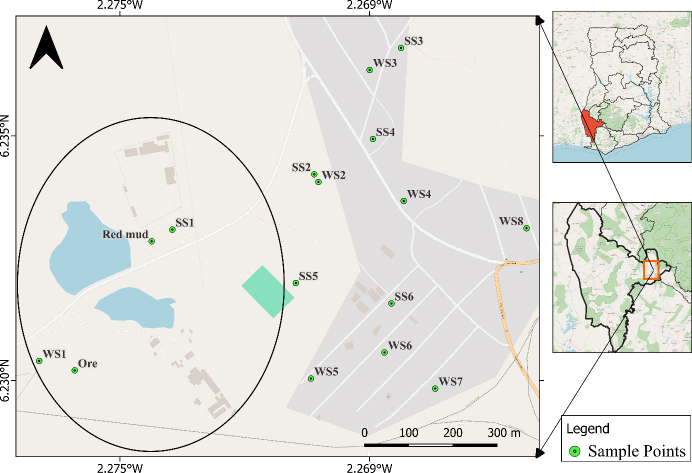
Geographical location and sampling points. WS represents water samples, and SS represents soil samples.
2.2.2. Preparation of Samples for Laboratory Analysis
The soil samples were first placed on metallic trays and air-dried for 7 days and then ground using a steel ball mill into fine particles and sieved with a 500 μm mesh size to have a uniform matrix size. The sieved soils were then placed in the oven to dry for 3 hours at 105 °C to completely remove excess moisture from the sample. The samples were then transferred to 500 ml labeled Marinelli beakers and tightly sealed and stored for 30 days, allowing short-lived daughters of radionuclides for both the 238U and 232Th decay series to attain secular equilibrium with their long-lived parent radionuclides.
3. EXPERIMENTAL
3.1. Instrumentation and Calibration
To obtain both qualitative and quantitative results from the samples, energy and efficiency calibration were performed for the analytical technique used, a high-purity germanium gamma detector, before the analysis. The calibration was performed using known test sources and mixed radionuclide standards for energy and efficiency to get accurate results. For efficiency calibration, the IAEA reference materials of IAEA-RGK-1 (K-ore), IAEA-RGU-1 (U-ore), and IAEA-RGTh-1 (Th-ore) were counted for 24 hours, and the spectrum was obtained and analyzed for the peak areas to form. The calculation was performed to obtain the net count from the sample by subtracting the raw net area from the background (IAEA 1989 and Martin, J. E. 2006). The resolution of this system is 1.96 keV of Co-60 (1332 keV) with a relative efficiency of 42.6%. Genie-2000 Gamma-Ray Acquisition and Analysis Software was used to identify the radionuclides present in the sample according to their well-known gamma energies. For each sample, the natural radionuclide concentrations of 226Ra, 232Th, and 40K were identified. Since 226Ra and its offspring are responsible for 98.5 percent of the radiological impacts of the uranium series found in nature, its precursors were disregarded. As a result, 226Ra rather than 238U was regarded as the reference for the 238U series. The counting time was enough to determine the energy lines of reference with the Minimum Detectable Activities (MDA) for all radionuclides, corresponding to 0.4, 1.1, and 1.4 Bq kg-1 for 226Ra, 232Th, and 40K, respectively at gamma-ray energy lines of 351.9 keV (36.6%) and 295.2 keV (18.5%) connected with the decay 214Pb, as well as at energies of 609.3 keV (46.1%) and 1120 keV (15%) related to the decay 214Bi, 226Ra activity concentration was calculated. The gamma rays with an energy of 911.1 keV (29%) associated with the decay of 228Ac, 583.1 keV (84.5%) related to the decay of 208Tl, and 238.6 keV (43.6%) associated with the decay of 212Pb were used to determine the activity concentration of 232Th. The gamma-ray with an energy of 1460.9 keV was used to calculate the activity concentration of 40K (10.67%). The actual samples were counted in the same geometry as the calibration standard and at the same counting time.
3.2. Calculation of Activity Concentration
The following expression was used to calculate the activity concentrations for natural radionuclides in soil, water, ore, and red mud samples (eq. 1):
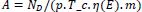 |
(1) |
where, A is the activity concentration, ND is the net count area of the radionuclides in the sample, p is the gamma-ray emission probability, η(E) is the absolute counting efficiency of the high-purity Germanium detector system,T_c is the time taken for the sample to be counted, and m is the mass of the sample.
3.3. Calculation of Absorbed Dose Rate
The equation (2) below was used to determine the absorbed dose rate from soil samples (Inayatullah et. al. 2016).
 |
(2) |
where, Dγ is absorbed dose rate, DCF is dose conversion factors; 0.462, 0.0417 and 0.604 (nGyh-1/Bqkg-1) respectively for 226Ra, 40K and 232Th, and A is Activity concentration of the radionuclide.
3.4. Calculation of Annual Effective Dose
To estimate the annual external effective dose, three factors were taken into consideration, i.e., the conversion coefficient from absorbed dose in the air to an effective dose; the outdoor occupancy factor of 0.2, which assumes an average semi-urban-rural lifestyle of the inhabitants where most adults spend their time in the farms away from the mine and the community, with children in schools most often; the number of hours per year (8760 h) and the annual estimated average effective dose received by a member of public is calculated using a conversion factor of 0.7 SvGy-1, given as follows (UNSCEAR 2000) (eq. 3):
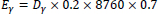 |
(3) |
Where, Eγ is the annual effective dose and Dγ is the absorbed dose rate in soil.
3.5. Committed Effective Dose
The committed effective dose of adult persons by water ingestion is determined by the activity concentration of 40K, 226Ra, and 232Th in the sample, and also the amount of water a person takes in liters per year. According to WHO guidelines for drinking water (WHO 2004), an individual is recommended to take 730 Ly-1, and the ingestion dose coefficient for 226Ra, 232Th, and 40K is 280, 230, and 6.2 nSvBq-1, respectively (UNSCEAR 2000).
The equation (4) below is used to calculate the committed effective dose.
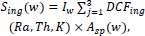 |
(4) |
where, Sing(w) is the committed effective dose, Asp is the activity concentration of the radionuclides in the sample in Bq.l-1, Iw is the intake of water in Ly-1 and DFCing is the ingestion dose coefficient in SvBq-1.
4. RESULTS AND DISCUSSION
Table 1 indicates the activity concentrations of 226Ra, 232Th, and 40K radionuclides present in the soil samples, bauxite ore, and red mud collected at the Awaso bauxite mining zone where the actual mining and processing takes place.
As shown in Table 1, the activity concentrations due to terrestrial gamma rays from 226Ra, 232Th, and 40K in the red mud were recorded as (45 ± 5) Bq kg-1, (64 ± 7) Bq kg-1, and (125 ±19) Bq kg-1 respectively. The values for both 226Ra and 232Th are above the world median values of 35 and 30 Bqkg-1, while the 40K figure is reported to be low as compared to 400 Bq kg-1 reported in UNSCEAR publication (UNSCEAR 2000).
| Sample ID | Radionuclide Concentration (Bq kg-1) | ||
|---|---|---|---|
| 226Ra | 232Th | 40K | |
| SS1 | 29 ± 3.2 | 32 ± 3 | 179 ± 18 |
| Red mud | 45 ± 5 | 64 ± 7 | 125 ± 19 |
| Ore | 39 ± 4 | 97 ± 11 | 15 ± 2 |
| Average | 38 ± 9 | 65 ± 32 | 106 ± 84 |
Table 2 is also a representation of values for radionuclide activity concentration (226Ra, 232Th, and 40K) in the entire soil samples as collected at the six different locations within the Awaso township away from the center of mining activities in the study area. The activity concentrations at SS1 and SS5 are higher compared to the rest of the sampled location points. As indicated in Fig. (1), samples SS1 and SS5 locations are closer to the mine, whilst the rest of the locations are spaced further away from the mine site. The results obtained for the red mud and bauxite ore indicate that the values for 226Ra and 232Th are higher in them, whilst 40K is lower in both samples. All the activity concentrations found in the soils from the study area are lower than the average activity concentration found in soil samples around the world, which is 35, 30, and 400 Bq kg-1 for the radionuclides mentioned and higher for ore and red mud (UNSCEAR 2000).
| Sample ID | Radionuclide Concentration (Bq kg-1) | ||
|---|---|---|---|
| 226Ra | 232Th | 40K | |
| SS2 | 17 ± 2 | 16 ± 2 | 56 ± 5 |
| SS3 | 13 ± 2 | 14 ± 2 | 15 ± 2 |
| SS4 | 12 ± 1.3 | 14 ± 2 | 106 ± 12 |
| SS5 | 26 ± 3 | 29 ± 3 | 167 ±16 |
| SS6 | 11 ± 2 | 10 ± 2 | 96 ± 10 |
| Minimum | 11 ± 2 | 10 ± 2 | 15 ± 2 |
| Maximum | 26 ± 3 | 29 ± 3 | 167 ±16 |
| Average | 16 ± 6 | 16 ± 7 | 88 ± 57 |
As depicted in Table 3, the absorbed dose rate for the soils in the mining enclave is higher than that for the red mud and the bauxite ore, considering the higher radionuclide levels present in the sample, especially 40K and their dose conversion factors. The arithmetic mean for the absorbed dose for the samples sampled within the Awaso community (soils) is (21 ± 3) nGyh-1, which is the potential energy deposited into tissue with respect to exposure to ionizing radiation. The arithmetic mean for the absorbed dose for the samples sampled within the mining zone (soil, bauxite, and red mud) is (61 ± 4) nGyh-1. Thus, the potential exposure is higher within the mining zone than the exposure in the community.
It is also illustrated in Table 3 that the annual effective dose was calculated for the samples, and it is observed that the dose for bauxite ore is higher than the one for the red mud and the soils. The calculated annual effective dose for the samples within the mine is 75 ± 5 µSv. The calculated annual effective dose for the samples within the community is 26 ± 3 µSv. These numbers are below the recommended dose limit of 20 mSv for workers and 1 mSv for members of the public, as stated by (ICRP 2007) in radiation protection principles for public radiation exposure control.
Using a radar plot, as shown in Fig. (2), allows for an intuitive visual comparison of absorbed doses and annual effective doses across different soil types or materials from the mining zone and the average soil within the township. This shows that soil from the mining zone has potential radiation exposure risks compared to soils within the township especially if considered as a building material in any form or shape.
| Location | Sample ID | Absorbed dose rate (nGyh-1) | Annual effective dose (µSvy-1) |
|---|---|---|---|
| Mining Zone | SS1 | 40 | 50 |
| Red mud | 65 | 79 | |
| Ore | 78 | 95 | |
| Average | 61 ± 4 | 75 ± 5 | |
| Awaso Township | SS2 | 20 | 24 |
| SS3 | 15 | 18 | |
| SS4 | 18 | 22 | |
| SS5 | 36 | 45 | |
| SS6 | 15 | 16 | |
| Minimum | 15 | 16 | |
| Maximum | 36 | 45 | |
| Average | 21 ± 3 | 26 ± 3 |
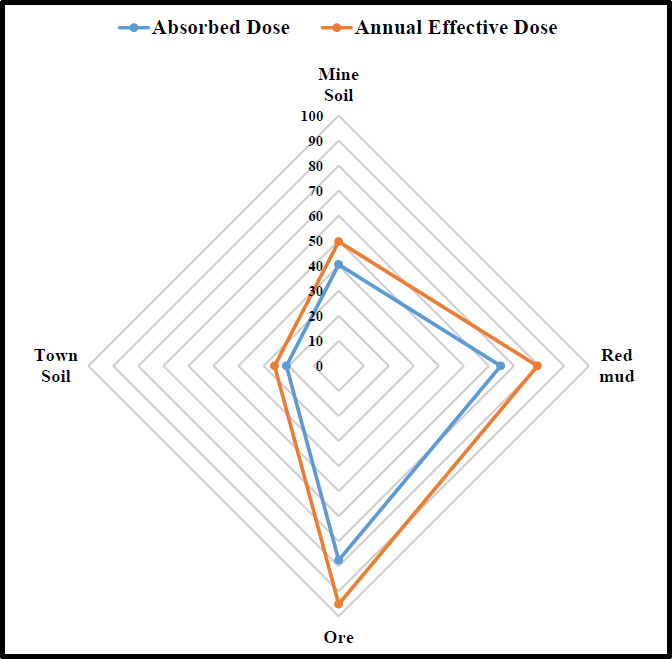
Comparison of risk of dose from materials within the mining zone and average soil within the Awaso town.
Table 4 illustrates the comparison of activity concentrations of the bauxite ore in this work to those published by other investigations in different locations across the world. The activity concentrations recorded in this study are lower than what is reported from the countries representing some of the regions of the world. The overall effect is that bauxite ore contains higher radionuclides, 226Ra, 232Th, than the world average (UNSCEAR 2000). On the other hand, the activity concentration of 40K for other studies, including Ghana, is within the world average figure of 400 Bq kg-1, though it was not detectable in some countries (UNSCEAR 2000). The results obtained for bauxite ore indicate that the activity concentrations of 226Ra, 232Th, and 40K were (39.42 ± 4) Bq kg-1, (97 ± 11) Bq kg-1, and (15 ± 2) Bq kg-1, respectively, in this study. It is also evident from this study that the activity concentration for both 226Ra and 232Th was above the world average.
| Activity Concentration (Bq kg-1) | Country | References | ||
|---|---|---|---|---|
| 238U | 232Th | 40K | ||
| 70.2 | 499 | - | Egypt | (Abbady A.G. et. al. 2006) |
| 64 | 154 | 9.4 | Brazil | (Righi, S. et. al. 2009) |
| 72.8 | 185 | 43 | Greece | (Karagiannidi et. al. 2005) |
| 150 | 205 | 28.3 | Greece | (Papatheodorou, G. et. al. 2005) |
| 419 | 256 | 47 | Hungary | (Somlai, J.) et. al. 2008 |
| 370 | 400 | 63 | China | (Turhan, S. et. al. 2011) |
| 15.5-405 | 19.9-414 | 12.8-111.6 | Turkey | (Cuccia, V. et. al. 2011) |
| 83.7 | 107.3 | 192.0 | S. Arabia | (Saleh, A. et. al. 2017) |
| 39 ± 4 | 97 ± 11 | 15 ± 2 | Ghana | Present study |
Results of Water Samples from the Study Area
Eight groundwater samples were analyzed to determine the radionuclides present, and the results obtained are presented in Table 5.
The results of 226Ra, 232Th, and 40K for the water samples were put into two segments, one representing samples from the mine concession and the other from the Awaso community. The activity concentration in the water sample from the mine of 226Ra, 232Th, and 40K is (6 ± 1) Bql-1, (7.4 ± 0.4) Bql-1, and (13 ± 0.3) Bql-1, respectively, and the committed effective dose is calculated to be 1.5 mSv. The average activity concentrations in the water samples sampled from the Awaso community for 226Ra, 232Th, and 40K are (1 ± 0.9) Bq l-1, (3 ± 1) Bql-1, and (16 ± 5) Bql-1 respectively. The results show that the mining activities have a direct influence on the increased 226Ra and 232Th activity concentration of the water within the concession as compared to the average activity concentrations in water sampled from the community. The results also show that the activity concentration for 226Ra is lower than the activity recommended by World Health Organization (WHO) guidelines in drinking water standards of 10 Bql-1 for both sampling segments. The activity concentration for 232Th is higher than the value of 1 Bql-1 as per standard (WHO 2004) in water samples from the mine and lower than the average concentration for water sampled for the community. The high activity concentration of 232Th recorded in the mine water is an indication that bauxite ore is more associated with the thorium series than any other radionuclide series, as recorded in Table 1. The WHO guidelines for drinking water do not recommend 40K because the body is capable of regulating it.
| Sample ID | Radionuclide Concentration (Bql-1) | Committed effective dose (mSv) | ||
|---|---|---|---|---|
| 226Ra | 232Th | 40K | ||
| WS1 | 6 ± 1 | 7 ± 0.4 | 13 ± 0.3 | 1.52 |
| Awaso Community | ||||
| WS2 | 1.5± 0.6 | 3 ± 0.5 | 15 ± 0.3 | 0.58 |
| WS3 | ND | 3 ± 0.3 | 14 ± 0.3 | 0.57 |
| WS4 | 1.2 ± 0.8 | 4 ± 0.4 | 14 ± 0.3 | 0.72 |
| WS5 | 1 ± 0.3 | 4 ± 1 | 2 ± 0.2 | 0.75 |
| WS6 | 1 ± 0.6 | 3 ± 0.4 | 18 ± 0.3 | 0.57 |
| WS7 | 0.8 ± 0.4 | 3 ± 0.5 | 16 ± 0.3 | 0.58 |
| WS8 | 0.6 ± 0.3 | 3 ± 2.1 | 12 ± 0.4 | 0.62 |
| Average* | 1 ± 0.9 | 3 ± 0.6 | 16 ± 5 | 0.63 ± 0.08 |
It is observed that WS1 has the highest committed effective dose of any other water sample onsite. The value for the committed effective dose depends on the activity concentration of the radionuclides, water consumption in liters per year, and the ingestion dose coefficient. The average committed effective dose for the Awaso community water samples is calculated to be (0.63 ± 0.08) mSv.
CONCLUSION
The different radionuclides that were found to be connected with NORMs in bauxite mining were discovered to be 226Ra, 232Th, and 40K. The level of exposure was determined by analyzing soil samples, bauxite ore samples, red mud samples, and water samples collected at the location where the study was carried out. According to the findings of the study, the levels of radioactivity in the soil for 226Ra, 232Th, and 40K at all of the locations within the mine and the surrounding communities where soil samples were collected were well within the threshold for the global average activity concentration of soil. The activity concentration that was found to be present in red mud and the bauxite ore was found to be higher than the concentration averagely expected to be present in the soil. The levels of radioactivity in the water were found to be within the average range for the world for 226Ra, 232Th, and 40K, and the committed effective dosage was calculated and compared to the world averages, respectively. In general, the results that were achieved for this study are comparable with the results that were obtained for other studies, such as those carried out in bauxite mines (UNSCEAR 2000 and WHO 2004). It also shows that the level of natural radioactivity in the soil, water, bauxite ore, and red mud in the study area is not considerable. As a result, it does not represent any significant risk of exposure to the general public or the personnel who work in the study region. The current data can be used to demonstrate that the bauxite mining operation does not significantly increase the activity concentration of 226Ra, 232Th, and 40K beyond normal values. This can be interpreted as an indirect demonstration that the bauxite mining operation does not significantly increase the annual dose received by the Awaso community.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design: OKA, data analysis and interpretation of results: MM, draft manuscript: JTB. All authors reviewed the results and approved the final version of the manuscript.
AVAILABILITY OF DATA AND MATERIAL
All the data and supporting information are provided within the article.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The research work is supported by the International Atomic Energy Agency (IAEA) through the Postgraduate Education Certificate program (PGEC), the Ghana Atomic Energy Commission (GAEC), and the School of Nuclear and Allied Sciences, University of Ghana. The authors would like to thank the technicians in the Food and Environmental Laboratory of the Radiation Protection Institute, GAEC, for their laboratory assistance in sample preparation and analysis.


