All published articles of this journal are available on ScienceDirect.
Larvicidal Potency of Ashes of Two Insecticidal Plants against the Activities of Anopheles coluzzii and Culex quinquefasciatus Mosquitoes
Abstract
Introduction
Insecticidal plants are widely studied materials that have intense applications in various fields of vector, pest, and disease control. They are among the recommended strategies to tackle the already established resistance in mosquitoes causing prevailing diseases in the world, especially in Africa.
Aim and Objective
The study aimed to assess the biological potency of ashes of scent leave (Ocimum gratissimum) and lemon grass (Cymbopogon citratus) against the larvae of Anopheles and Culex mosquitoes.
Methods
Larvae of mosquitoes were sourced, and plant ashes were prepared and exposed to 20 larvae of both mosquitoes in 1g, 2.5g, 5g, 10g, and 15g concentrations. Treatment concentrations were formulated by mixing ashes in 100ml of water in triplicate. Mortality, acute toxicity, and sub-chronic toxicity data were obtained.
Results
Mosquito larval mortality increased with time at all concentrations of test plants, and sub-chronic toxicity showed complete mortality in all treatments. Acute toxicity of Culex larvae was highest in 15g of lemon grass and complete mortality was recorded after 30 minutes of exposure. There was no acute toxicity recorded with scent leave exposure. The Lethal Dose (LD50) for Anopheles mosquitoes recorded with scent leave ashes was 0.319g (y=1.928x+0.96; R2=0.221, p= 0.407), and for Culex mosquitoes, it was recorded to be 0.424g with lemon grass exposure (y=1.86x+0.69; R2=0.221, p= 0.240). Remarkably, lemon grass at a concentration of 1.250g and 3.247g caused 95% toxicity in Anopheles (y=15.85x-0.25; R2=0.633, p> 0.05) and Culex (y=2.918x-0.18; R2=0.388, p= 0.254) mosquitoes, respectively. LT50 of mosquitoes was between 21.3 minutes and 1451.4 minutes, whereas LT95 was between 37.1 minutes and 1740.4 minutes, respectively. No adult mosquito emergence was recorded.
Conclusion
Ashes of both plants, especially lemon grass, could be considered better materials for local treatment of the mosquito breeding sites.
1. INTRODUCTION
Insecticidal plants (bio-insecticides) are widely studied alternative materials with intense applications in various fields of entomology. Mosquitoes of the genera Anopheles, Culex, and Aedes are problematic species causing diseases of public health importance. Mosquitoes are flying insects (Diptera: Culicidae) with a greater potential to cause disease in the African region, especially in Nigeria (Caglioti et al., 2013). Worldwide, in 2019, there were documented 56 million dengue cases, over 227 million cases of malaria, and over 400 thousand deaths caused by malaria (WHO, 2020; Du et al., 2021). According to the World Health Organization (WHO, 2005), Nigerians suffer over a quarter of the global malaria burden, leading to several degrees of mortality, morbidity, and risk factors (Dawaki et al., 2016; Obasohan et al., 2020). Other associated African countries in the West African region are not exempted from mosquito-borne diseases. Arthropod-borne viruses are equally not excluded from the many diseases caused by mosquitoes. Controlling mosquitoes using chemical insecticides has resulted in resistance of disease-causing species. Failure in the effectiveness of recommended control measures is the principal cause of hyper-expression of diseases worldwide. Several methods of mosquito intervention include physical, chemical, and biological approaches. Active collection of species and sometimes over-collection, destruction of potential larval breeding sites, and technologies for passively trapping adult mosquitoes have been highlighted (Benelli et al., 2016). Biological methods are among the best, cheapest, safest, and easiest methods. Biological intervention involves the use of microbial agents as well as potent insecticidal plants. Bacteria (Bti and Bs) and several entomopathogenic fungi have been reported as biological agents as they do not contain any harmful chemicals (Sabbahi et al., 2022).
Chemical insecticides are well acknowledged to be the only recommended vector intervention known to date. Insecticides recommended for innovative applications have been classified by WHOPES in 2020 into pyrethroids, organophosphates, carbamates, and organochlorides. Amongst these, pyrethroids have been one of the most commonly used due to their low effect on human health and insecticidal activity, as well as their global applications in different insecticidal sprays. The continuous use and, sometimes, the application of inappropriate dosage leads to the buildup of resistant population, a problem the WHO African region is faced with today (Tudi et al., 2022). The major cost-effective intervention measure today is the use of Long-lasting Insecticidal Nets (LLINs). Different countries have different use percentages of LLINs, but the issues of mosquitoes, especially the diseases they transmit, are still prevailing. The deployment of chemical intervention may be complicated in terms of access in all areas, time-consuming in management of insecticide resistance when it builds up, expensive chemicals, as well as not being suitable for onsite applications, where vulnerable populations, such as children under the age of five and pregnant women, are dwelling. In insecticide spraying inside houses, commercial spraying tools and chemicals are used and expertise is required. In the past, spraying of insecticides indoors was effective in some locations (Corrêa et al., 2019), but their sustainability suffered setbacks. So, it is necessary to take into account the potency of the available interventions while designing novel techniques that mosquitoes would be susceptible to. Likewise, newer techniques can accurately identify the best application approaches and detect the possible concentrations with low mammalian toxicity that would not affect non-targeted organisms when applied in the ecosystem. Other techniques besides the use of insecticides have been detailed in the literature (Nalinya et al., 2022). Scientists are persistent in discovering a long-lasting solution to mosquito-borne diseases.
Recently, the need to discover bioactive substances with the potential for industrial production has increased, because the available interventions targeting insect pests and vectors of diseases are failing. Most insecticidal plants are locally available in the environment as weeds and are underutilized. Sometimes, they are first discovered for the aroma they produce, which then triggers the need to try their potency. Aromatic plants are more targeted in trial studies than non-aromatic plants. This may be the major challenge affecting the discovery of active insecticidal plants in mosquito-endemic regions. In this scenario, appropriate selection is required to determine which portions of the plant have superior bioactive components or which species of microbial population is more active than the others (Vaou et al., 2021).
Two methods are very important in the fight against mosquitoes, targeting the larval and the adult populations. Presently, different formulations of insecticidal plants are being used, such as essential oils, dried grounded plants into powder or dust, and bio-ashes. Severe toxicity of botanicals has been reported; however, their safety has equally been highlighted for the environment, where diverse non-target population species are exceedingly present (Abok et al., 2018; Ombugadu et al., 2020; Pam et al., 2021; Aliyu et al., 2022). The leaves of scent leave (O. gratissimum) and lemongrass (C. citratus) contain many compounds that exhibit insecticidal properties and are able to cause mortality or physiological stress in mosquito species (Plata-Rueda et al., 2020). C. citratus is a plant that is widely distributed in tropical and subtropical parts of Africa, Asia, and America (Boukhtaem et al., 2014). The plant has tremendous commercial value for the essential oils that are utilized in traditional medicine and culinary technology (Mirghani et al., 2012). Traditional uses of scent leaf (O. gratissimum L) raw extract include the treatment of epilepsy, high fever and diarrhea, fever, cold, catarrh, and fungal infections. It has also been shown to be effective in the management of storage crop pests (Okwuonu et al., 2023). Because there are no reports on the potency of bio-ashes of insecticidal plants in the literature and toxicity of non-target species has not been recorded as a control against the activities of mosquitoes, this study became necessary.
Therefore, this study aimed to determine the bio-toxicity of two insecticidal plants against the activities of mosquito larvae in Ethiope East LGA. This study could benefit local residents in the utilization of local ashes of these plants as well as its broad adoption in other areas of Africa where they are commonly available. Furthermore, the importance of packaged insecticidal ashes cannot be underestimated, especially with the resurging issues of insecticide resistance in the African region (Ojianwuna et al., 2022; 2022a).
2. MATERIALS AND METHODS
2.1. Study Location
The experiment was set up in the exposure room located in the animal house of the Department of Animal and Environmental Biology, Delta State University, Abraka. Mosquitoes from their natural breeding sites were collected from five communities in Ethiope, East Local Government area, including Umeghe (Lat. 5.825528oN and Long. 6.123437oE), Oviore-Ovu (Lat. 5.657178oN and Long. 5.924573oE), Oria (Lat. 5.761845oN and Long. 6.055379oE), Abraka main town (Lat. 5.785923oN and Long. 6.116988oE), and Ugono (Lat. 5.771618oN and Long. 6.135377oE). Mosquitoes collected from the various communities were allowed to acclimatize to the laboratory condition for 24 hrs at 27 ± 2°C and relative humidity of 77 ± 2% using an electrified humidifier. 12:12 photoperiod was maintained.
2.2. Source and Preparation of Test Materials
Fresh leaves of scent leave plant (O. gratissimum) and lemongrass (C. citratus) were locally sourced from within the locality. These leaves were air-dried on the laboratory long bench at room temperature, and 700g of each plant was separately placed in two vessels to burn to ashes. The ashes of each plant were retrieved and stored in an air-tight container.
2.3. Collection and Rearing of Mosquitoes
Wild strains of Anopheles and Culex larvae and pupae were collected from various breeding habitats in the study location using ladles, scooping spoons, sieves, transparent buckets, and pipettes. Ladles and scooping spoons were used to thrust into the natural habitat of mosquitoes and a pipette was used to remove predators of mosquito larvae. Sampling was done in the early hours of the morning (7:30-10:30 am). Mosquitoes were commonly encountered in ditches, puddles, tire marks, plastic containers, ponds, and many other potential habitats, as observed by Ojianwuna and Enwemiwe (2021a). Mosquitoes collected were transported to the insectary of the animal house and reared in the laboratory following standard rearing protocols till species attained the third instar larvae in the case they did not.
2.4. Experimental Design
The experiment was conducted using a 500ml capacity vial, replicated into three, and labelled for each concentration of test plants as Lemon Grass (LG) and Scent Leave (SL). Test plants were measured in grams, including 1g, 2.5g, 5g, 10g, and 15g, respectively, following a previous exposure performed by Ojianwuna et al. (2021a). These plants were emptied into 100ml of water to form 0.01g/ml, 0.025g/ml, 0.05g/ml, 0.1g/ml, and 0.15g/ml of the test materials. 20 active third instar larvae of mosquitoes were introduced into the setup and mortality readings were recorded at 5 min, 10 min, 15min, 20min, 30min, 40min, 50min, 60min, and 6hrs, and thereafter, readings of mortality were taken for 24hrs and every 24hrs until all the mosquitoes were either dead or emerged. The larvae were checked from time to time for acute and sub-chronic mortality. The larvae were considered dead after being prickled with a plastic pipette and subsequently removed. Larvae were fed with biscuit and yeast mixture (1 stick of biscuit; 10 tablets of yeast) daily.
2.5. Mosquito Identification
After exposure to treatments, mosquitoes in the control group of the experiment were killed and morphologically identified using the Anopheles key by Coetzee (2020) and the Culex key by Rueda (2004). Mosquitoes were further preserved in Eppendorf tubes filled with silica gel and molecularly identified. The mosquitoes were DNA extracted and PCR amplified following detailed description and protocol by Wilkins et al. (2006) and Egbedegbe et al. (2023). The species-specific identification of Anopheles mosquitoes was performed using the primers as follows: Anopheles gambiae (5-GCTTACTGGTTTGGTCGGCATTG-3), Anoph- eles merus (5-CAACCCACTCCCTTGACGATG-3), An. quadriannulatus (5-GCATGTCCAAGATGGTTCGCTG3), Anopheles arabiensis (5-GTGTTAAGTGTCCTTCTCCGTC- 3), Anopheles coluzzii (M form; 5TAGCCAGCTCTTGT CCACTAGTTTT-3), Anopheles sensu stricto (S form; 5-CCAGACCAAGATGGTTCGCTG-3). Culex mosquitoes were DNA extracted and PCR amplified following the guidelines by Livak (1984) and Smith and Fonseca (2004) using three primers of ACEquin (5′CCTTCTT GAATGGCTGTGGCA-3′), ACEpip (5′-GGAAACAACGACGTA TGTACT-3′), and B1246s (5′TGGAGCCTCCTCTTCACGG-3′).
2.6. Statistical Analysis
Mosquito Larval Mortality (MLM) was computed using the following formula:
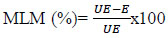 |
Where, UE is the percentage of mosquitoes in the unexposed group and E is the percentage in the exposed group.
Mortality data were entered into an MS Excel spreadsheet and carefully checked for entry errors. Descriptive statistics, including means, standard errors, percentages, and line charts, were used in data presentations and computed using the XL STAT 2023 version. The Analysis of Variance (ANOVA) test was used to check for significant differences set at α= 0.05. The probit model was used to analyze Lethal Dosage (LD50 and LD95) as well as Lethal Time (LT50 and LT95). Multivariate analysis was performed to check for the relationship between lethal time and time.
Mosquito Emergence (ME) was computed using the WHO formula (WHO, 2005):
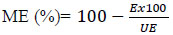 |
Where, E = percentage of mosquitoes that survived or emerged in the exposed group and UE= percentage of mosquitoes that survived or emerged in the unexposed group. Mosquito emergence was carefully observed in the unexposed group, and where it was less than 80%, the experiment was repeated. However, Abbott’s formula was used to correct mosquito emergence between 80 and 95% in the unexposed group.
3. RESULTS
3.1. Mosquito Larvae Mortality and Adult Emergence
The acute toxicity bioassay of ashes of two insecticidal plants against mosquito activities in Ethiope East LGA, Delta State, Nigeria, is shown in Table 1. There was a slight increase in larval mortality as concentration increased with time. Culex mosquitoes were more susceptible to the ashes of lemon grass as 15g of the test plant caused the highest toxicity. Larval mortality was equally high in Anopheles mosquitoes exposed to scent leave. Anopheles mosquitoes exposed to 1g of lemon grass resisted the ashes and recorded the lowest mortality. Culex mosquitoes exposed to 1g scent leave plants equally exhibited low mortality. The differences between Culex and Anopheles mosquitoes exposed to scent leave and Anopheles mosquitoes exposed to lemon grass were not significant (p>0.05) (Table 1). However, there was a significant difference in Culex mosquitoes exposed to lemongrass (p<0.05). No adult emergence as a result of exposure was recorded in this study.
3.2. Time Susceptibility
The time susceptibility of mosquito larvae exposed to ashes of scent leave and lemon grass is presented in Table 2. Toxicity increased in both mosquitoes exposed to the ashes of the insecticidal plants. Complete mortality was recorded in all ashes of scent leave and lemon grass at different times. In Anopheles mosquitoes exposed to the treatment ashes, no mortality was recorded in 1g and 2.5g of scent leaves between 5 minutes and 50 minutes of exposure. Similarly, no mortality was recorded in 5g, 10g, and 15g of scent leaves between 5 minutes and 30 minutes. All Anopheles larvae died at 6 hours of post-exposure. For lemon grass, no mortality was recorded in 1g between 5 minutes to 6 hours, in 2.5g between 5 minutes to 50 minutes, in 5g and 10g, respectively, between 5 minutes and 40 minutes, and in 15g between 5 and 30 minutes. The mortality of Anopheles larvae with 5g, 10g, and 15 g of lemon grass was recorded at 6 hours, whereas Anopheles larvae mortality with 2.5g and 1g of the treatment was recorded at 24 hours and 48 hours, respectively. The differences between the time mortality were significant (P<0.05) (Table 2).
| Mosquito | Concentration (grams) | Log Concentration | SL Mean Mortality | LG Mean Mortality |
|---|---|---|---|---|
| Culex | 0.00 | 0.000 | 0.00 ± 0.00A | 0.00 ± 0.00A |
| 1.00 | 0.000 | 10.72 ± 7.74B | 19.1 ± 8.54C | |
| 2.50 | 0.398 | 12.72 ± 8.66B | 22.18 ± 8.92CD | |
| 5.00 | 0.699 | 14.54 ± 9.76BC | 27.82 ± 9.24F | |
| 10.0 | 1.000 | 14.54 ± 9.76BC | 48.54 ± 11.24H | |
| 15.0 | 1.176 | 14.72 ± 9.74BC | 55.82 ± 9.40I | |
| Anopheles | 0.00 | 0.000 | 0.00 ± 0.00A | 0.00 ± 0.00A |
| 1.00 | 0.000 | 24.92 ± 11.1D | 10.18 ± 7.56B | |
| 2.50 | 0.398 | 26.18 ± 11.3DE | 21.28 ± 10.66CD | |
| 5.00 | 0.699 | 27.10 ± 10.7E | 24.54 ± 10.92D | |
| 10.0 | 1.000 | 27.28 ± 10.8E | 26.18 ± 10.84E | |
| 15.0 | 1.176 | 32.36 ± 10.7F | 29.64 ± 5.47G |
| Treatment | Concentration (g) | 5 | 10 | 15 | 20 | 30 | 40 | 50 | 60 | 6hr | 24hr | 48hr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | 1 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 25.50DEFGH | 60.0J | 60.0J | 60.0J |
| 2.5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 36.00GHI | 60.0J | 60.0J | 60.0J | |
| 5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 6.00ABC | 10.50ABCD | 27.00EFGH | 60.0J | 60.0J | 60.0J | |
| 10 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 4.50ABC | 10.50ABCD | 30.0FGH | 60.0J | 60.0J | 60.0J | |
| 15 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 18.00CDEF | 28.50FGH | 40.50HI | 60.0J | 60.0J | 60.0J | |
| LG | 1 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 24.00DEFG | 60.0J |
| 2.5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 13.50ABC | 17.00IJ | 60.0J | 60.0J | |
| 5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 6.00ABC | 16.50BCDEF | 60.0J | 60.0J | 60.0J | |
| 10 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 12.00ABCDE | 24.00DEFG | 60.0J | 60.0J | 60.0J | |
| 15 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 1.50A | 27.00EFGH | 36.00GHI | 60.0J | 60.0J | 60.0J |
| Treatment | Concentration (g) | 5 | 10 | 15 | 20 | 30 | 40 | 50 | 60 | 6hr | 24hr | 48hr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | 1 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 28.5EFG | 60.0J |
| 2.5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 45.0HIJ | 60.0J | |
| 5 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 60.0J | 60.0J | |
| 10 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 60.0J | 60.0J | |
| 15 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.50A | 60.0J | 60.0J | |
| LG | 1 | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 0.00A | 7.50ABC | 19.50BCDEF | 24.00DEF | 46.50IJ | 60.0J |
| 2.5 | 0.00A | 0.00A | 0.00A | 0.00A | 1.50A | 4.50AB | 15.00ABCDEF | 19.50BCDEF | 28.50EFG | 54.0IJ | 60.0J | |
| 5 | 0.00A | 0.00A | 0.00A | 4.50A | 7.50A | 13.50ABCDE | 19.50BCDEF | 22.50CDEF | 43.50GHI | 60.0J | 60.0J | |
| 10 | 0.00A | 0.00A | 4.50AB | 7.50ABC | 30.00FGH | 58.50IJ | 60.0J | 60.0J | 60.0J | 60.0J | 60.0J | |
| 15 | 0.00A | 10.50ABCD | 18.00BCDEF | 28.50EFG | 43.50GHI | 60.0J | 60.0J | 60.0J | 60.0J | 60.0J | 60.0J |
In Culex mosquitoes exposed to the treatments, mortality equally increased with time. No mortality was recorded with 1g, 2.5g, 5g, and 10g of scent leave ashes between 5 minutes and 6 hours. Mortality with 15g ashes started at 6 hours, and was completed at 24 hours. Mortality with 5g and 10g was completely recorded in 24 hours, while that of 1g and 2.5g ended in 48 hours. For lemongrass ashes, no mortality was recorded in 1g between 5 minutes and 50 minutes, 2.5g between 5 and 20 minutes, 5g between 5 minutes and 15 minutes, 10g between 5 minutes and 10 minutes, and 15g in 5 minutes. Mortality was completed in 15g and 10g of lemon grass ashes at 40 and 50 minutes, respectively. More so, mortality in 5g was completed in 24 hours, while in 2.5g and 1g of ashes, mortality was completely recorded in 48 hours, respectively. The differences between the time mortality were significant (P<0.05) (Table 3).
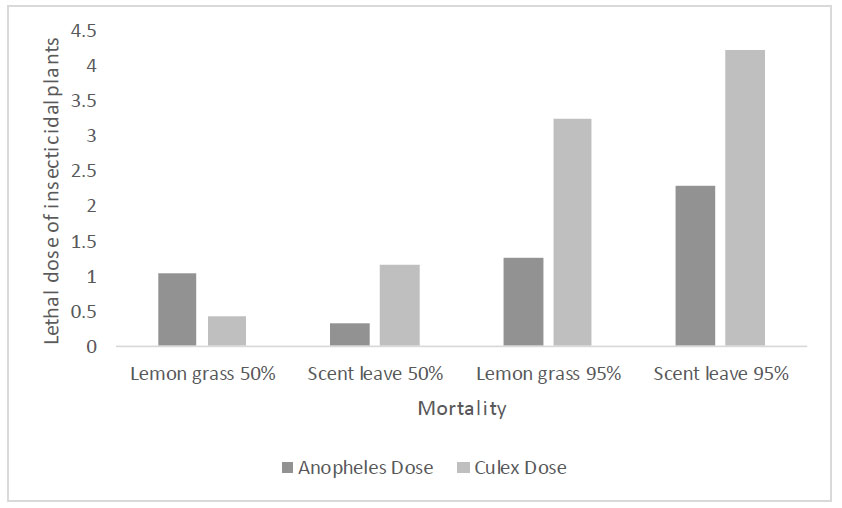
Lethal dose of insecticidal plants to which Anopheles and Culex mosquitoes have been exposed in Ethiope East LGA, Delta State, Nigeria.
3.3. Probit Model Analysis of Lethality
The logarithmic dose for the lethality of the ashes of the insecticidal plants to which Anopheles and Culex mosquitoes were exposed in Ethiope East LGA, Delta State, Nigeria, is presented in Fig. (1). The lowest dosage that could cause mortality in 50% of the mosquito larval population was recorded in Anopheles mosquitoes exposed to scent leave ashes (0.319g) (y=1.928x+0.96; R2=0.221, p= 0.407). Mortality of Culex mosquitoes (50% larval population) was recorded upon lemongrass exposure (0.424g) (y=1.86x+0.69; R2=0.221, p = 0.240). Remarkably, lemon grass of concentration 1.250g and 3.247g caused 95% toxicity in Anopheles (y=15.85x-0.25; R2=0.633, p> 0.05) and Culex (y=2.918x-0.18; R2=0.388, p= 0.254) mosquitoes, respectively. All the lethal doses were not significant, showing the model to be appropriately fitted.
The lethal time of mosquito larvae exposed to the ashes of the two insecticidal plants is shown in Table 4. Irrespective of the test plants, the lethal time for 50% of mosquitoes was between 21.3 minutes and 1451.4 minutes, whereas LT95 was between 37.1 minutes and 1740.4 minutes, respectively. Culex mosquitoes exposed to 15g of lemon grass ashes recorded the highest mortality in reduced time. A similar trend was observed for the same mosquito larval population exposed to 10g of lemongrass. All the lethal times of mosquito larvae exposed to the two insecticidal plants were significant (p<0.05) (Table 4), except for the lethal time of the Culex mosquitoes exposed to scent leave and Anopheles mosquitoes exposed to 1g and 2.5g of scent leave (p>0.05).
| Larvae | Treatment | Log Concentration (grams) | Regression Line | R2 (p-value) | LT50 | LT95 |
|---|---|---|---|---|---|---|
| Culex | Scent leave | 0.000 | y=0.006x-8.25 | 0.472 (>0.05) | 1451.0 (1228.4-1655.7) |
1740.4 (1555.2-1901.0) |
| 0.398 | y=0.006x-8.37 | 0.667 (>0.05) | 1332.6 (1198.0-1500.8) |
1594.6 (1338.1-1752.7) |
||
| 0.699 | y=0.006x-8.65 | 0.875 (>0.05) | 902.4 (788.0-1120.5) |
1241.3 (1005.8-1457.4) |
||
| 1.000 | y=0.020x-14.49 | 1.00 (>0.05) | 717.9 (520.8-912.7) |
799.3 (588.5-1125.4) |
||
| 1.176 | y=0.0142x-7.03 | 0.939 (>0.05) | 499.2 (281.6-605.8) |
616.0 (411.5-817.4) |
||
| Lemon grass | 0.000 | y=0.0017x-1.51 | 0.314 (<0.0001) | 877.1 (741.6-1063.1) |
1831.6 (1555.4-2241.5) |
|
| 0.398 | y=0.002x-1.35 | 0.332 (<0.0001) | 655.2 (545.5-809.0) |
1453.7 (1220.4-1807.5) |
||
| 0.699 | y= 0.003x-1.12 | 0.322 (<0.0001) | 337.5 (268.4-451.1) |
833.6 (662.5-1139.8) |
||
| 1.000 | y= 0.135x-3.83 | 0.780 (0.0002) | 28.4 (26.5-30.4) |
40.6 (37.6-44.9) |
||
| 1.176 | y= 0.103x-2.91 | 0.611 (0.0001) | 21.3 (19.3-23.3) |
37.1 (33.6-42.3) |
||
| Anopheles | Scent leave | 0.000 | y= 0.590x-35.61 | 0.877 (>0.05) | 60.3 (58.1-63.7) |
63.1 (60.4-66.9) |
| 0.398 | y= 0.636x-37.90 | 0.883 (>0.05) | 59.6 (56.4-62.3) |
62.2 (58.4-64.5) |
||
| 0.699 | y= 0.071x-4.41 | 0.743 (0.0002) | 61.8 (60.4-74.7) |
84.8 (75.2-104.9) |
||
| 1.000 | y= 0.082x-4.93 | 0.755 (0.0002) | 60.2 (56.6-66.3) |
80.3 (72.3-97.0) |
||
| 1.176 | y= 0.077x-3.97 | 0.683 (<0.0001) | 51.7 (48.8-55.2) |
73.1 (67.2-82.5) |
||
| Lemon grass | 0.000 | y=0.0053x-7.89 | 0.599 (0.994) | 1487.8 (1280-1619) |
1797.9 (1541-1984) |
|
| 0.398 | y=0.011x-2.76 | 0.838 (<0.0001) | 259.5 (227.8 - 296.6) |
414.3 (368.2-477.7) |
||
| 0.699 | y= 0.091x-5.99 | 0.831 (0.001) | 66.02 (61.2-79.9) |
84.1 (73.6-119.1) |
||
| 1.000 | y= 0.094x-5.80 | 0.788 (0.0004) | 61.7 (58.2-64.5) |
79.1 (71.2-98.1) |
||
| 1.176 | y= 0.098x-5.41 | 0.746 (0.0002) | 55.2 (52.6-58.6) |
72.0 (66.7 - 81.7) |
4. DISCUSSION
The findings presented in this study have demonstrated insecticidal plants of O. gratissimum and C. citratus to affect the ultimate survival of Anopheles and Culex mosquitoes in Ethiope East, Delta State. This may be because botanicals pose no risk to the environment or human health, and plant-based insecticides have long been touted as appealing substitutes to synthetic insecticides for the management of mosquitoes. Notable studies have pointed out the resistance of mosquitoes to selected pyrethroid insecticides, and their mixtures with other insecticides and synergists in Delta State (Ojianwuna et al., 2022a; Ojianwuna et al., 2021a). Another study has also highlighted the abundance and distribution of mosquito breeding habitat in an adjoining LGA with that reported in this study, at less than 5km (Ojianwuna et al., 2021). Overall, both insecticidal plants under study have acutely reduced the population of mosquito larvae, with an increase in concentrations further reducing the population. Neither concentrations caused complete mortality in the acute phase, regardless of the species of mosquitoes exposed to the test plants. When the experiment was observed closely in the sub-chronic phase, a greater proportion of mosquito larvae were knocked off, probably due to physiological stress caused by the active components of plants, primarily citronellal. These results, therefore, indicate that the ashes of these plants may broadly act as mortality materials when packaged in powdery forms for field applications. These plants have also shown broad efficacy for other insects (Okwuonu et al., 2023).
Considering that both plants caused mortality and reduced adult emergence, this may indicate them to be good plant materials for adult or even larval toxicity (Ojianwuna and Enwemiwe, 2021a); however, mortalities in the various test plant groups were slow and sub-chronic rather than acute. Nevertheless, several other studies have demonstrated scent leave and lemon grass to cause mortality in many insect pests (Ojianwuna and Umoru, 2011; Okwuonu et al., 2023), as observed here. In a laboratory trial, 1.0 and 1.5 grams of scent leave caused significantly high mortality against subterranean termites and even their mixtures with naphthalene and kerosene (Ojianwuna and Enwemiwe, 2021a). Other authors have evaluated these plants as potential agents in larval source reduction (Ombugadu et al., 2020; Pam et al., 2021; Plata-Rueda et al., 2020). Studies by Adakole and Adeyemi (2012), Ebe et al. (2015), and Unachukwu et al. (2016) have shown significantly high mortality against the activities of mosquitoes exposed to both plants in this study. Mariam et al. (2021) also reported great mortality of mosquito larvae with an increase in the concentration of C. citratus; this study has demonstrated the mosquitoes to have a high susceptibility to time mortality upon exposure to these plants, broadly corroborating the results of this study. These plants may not only affect the survival rate, but may have a prolonged influence on mosquito development. Given this discontinuity, further work is required to elucidate the underlying physicochemical and physiological factors that might influence the sub-chronic mortality observed in this study.
The amount of plant ashes per unit volume of water, and hence the species of mosquito larvae, was demonstrated here to be an important variable. Studies investigating the effect of plant materials and other potent substances on mosquitoes and other insect pests have utilized a range of concentrations; for instance, Ojianwuna and Enwemiwe (2022) used a range from 0.005% to 0.02%ml, whereas Abok et al. (2018) used 10mg/ml to 100mg/ml, and Ojianwuna et al. (2021b) used 0.05ml to 0.60ml. Also, the time of mortality associated with each experiment differed substantially; Abok et al. (2018) observed sub-chronic mortality at 72 hours and resistance to plants exposed to mosquito larvae between 24 and 48 hours. More so, Ojianwuna et al. (2021b) observed mortality of Culex mosquitoes exposed to several plants in 24 hours and high acute toxicity with the use of petroleum products in 30 minutes, which was insignificant with progressing life stages. The length of time for mortality to occur may relate to the effectiveness of the plant the mosquitoes are exposed to. A holistic study on the effect of these plants on the three vectors of disease is important. Therefore, future studies should consider including the three mosquito species to observe the susceptibility status of different species.
Larval exposure to the two plants in this study caused mortality at different times. Acute toxicity (between 10 minutes and 6 hours) in this present study was highest in Culex mosquitoes exposed to 15g of lemongrass. The present study demonstrated complete mortality in Culex mosquitoes exposed to ashes of lemon grass (15g) within 30 minutes of exposure, whereas in other treatments, complete mortality was observed between 6 hours and 48 hours of sub-chronic exposure. Culex mosquitoes exposed to scent leave were susceptible between 10 and 60 minutes, Anopheles mosquitoes exposed to scent leave were susceptible between 10 and 30 minutes, while Anopheles mosquitoes exposed to ashes of lemon grass were susceptible between 10 to 40 minutes; a similar trend was observed with scent leave exposure. The findings of this study in terms of time mortality are in accordance with studies previously carried out by Ojianwuna et al. (2021c), but the concentrations and the species of larvae differed between these studies. The reason for the high susceptibility in Culex mosquitoes may be due to the blockage of the siphon by suspending ashes, while in Anopheles, the reduced siphon may have been an advantage to their survival.
This study has also demonstrated the lethal dose of scent leaves ashes to cause the highest 50% lethality in Anopheles mosquitoes and lemon grass in Culex mosquitoes. Lemon grass caused 95% toxicity in both species. The lethal concentrations reported in this study have not been found to correspond to those recorded in the study by Ojianwuna and Enwemiwe (2021), where reduced concentrations were observed. This may be due to differences in species. Culex mosquitoes exposed to 15g of lemon grass ashes recorded the highest mortality in reduced time. A similar trend was observed in the same mosquito larval population exposed to 10g of lemongrass. The finding of this study on lethal time corroborates a previous study by Ojianwuna et al. (2022b), where Culex mosquitoes recorded the lowest lethal time with 1.5g of Psoralea corylitolia and Anopheles with 1.5g of Sesamum radiatum. There are no studies that have investigated the potential ecological impacts of using plant-based insecticides for mosquito control. This becomes necessary for future studies on bioinsecticidal discovery. Likewise, the effects of bioinsecticides on non-target species have been scarcely studied. A study by Giunti et al. (2022) observed respiratory efficiency, predation capacity, and many other natural enemy activities to be impacted in a field survey. While these alternatives may be safer for human health and the environment compared to synthetic insecticides, their long-term effects on non-target organisms and ecosystem dynamics need thorough evaluation. Understanding the mechanisms of action underlying plant-based insecticides' toxicity to mosquito larvae is crucial for optimizing their effectiveness and minimizing the risk of resistance development. Investigating factors, such as the bioavailability of active compounds, their interaction with mosquito physiology, and potential synergistic effects with other compounds, can provide valuable insights into how to enhance the efficacy of plant-based larvicides.
CONCLUSION
This research has demonstrated the effects of O. gratissimum and C. citratus on mortality and adult emergence, time mortality, and lethal time and concentration against Anopheles and Culex mosquito larvae. Both plants have been found to cause sub-chronic mortality with increased concentrations, eliciting further lethality in time and concentration. When mosquito larvae were exposed to a higher concentration of test plant ashes, a higher proportion (50% of the population) of Culex mosquitoes became susceptible to C. citratus and the same population of Anopheles mosquitoes was susceptible to O. gratissimum. However, across all assays, lemon grass caused 95% lethality in both species. These data suggest that both plants may cause physiological and developmental stress in mosquito larvae, leading to complete mortality. The findings of this study indicate that the application of insecticidal plants may not cause acute toxicity, but chronic toxicity. Therefore, promoting these plants in field application at local breeding sites by residents might alter the disease transmission dynamics of the given area.
AUTHORS’ CONTRIBUTION
CC, VN, and EE conceived and designed the experiment. EE, SI, DR, and SA performed field and laboratory experiments, and VN contributed to data entry and analysis. VN and CC interpreted the data, performed the literature search, and supervised the study. All authors have contributed to the writing and critical revision of the manuscript, and have approved the final version for submission.
REFERENCES
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link] [PubMed Link]


