All published articles of this journal are available on ScienceDirect.
Intra-reach Headwater Fish Assemblage Structure
Abstract
Large-scale conservation efforts can take advantage of modern large databases and regional modeling and assessment methods. However, these broad-scale efforts often assume uniform average habitat conditions and/or species assemblages within stream reaches.
Objective:
I examined fish species assemblage structure within two forested headwater stream reaches of the Great Lakes drainage to evaluate the validity of within stream uniformity.
Results:
Sample assemblages within stream reaches were not distinct, except for where habitat changed sharply from forest to wetland.
Conclusion:
The results support the general assumption that fish assemblages are uniform within stream reaches, for the purposes of coarse scale analyses, but more research is needed to test the consistency of these finding across all headwater streams of a watershed or region.
INTRODUCTION
The global connectedness of ecological systems and ecological problems is becoming ever more apparent, as are the associated complexities. As a result, effective large-scale conservation is relying more on regional modeling and assessment. These regional approaches allow for evaluation of resource conditions over large areas and often have multiscale capabilities allowing examination of conditions at finer scales, within limits (Prior-Magee et al. 2007, National Fish Habitat Board, 2010, McKenna and Johnson 2011, McKenna et al. 2015). Data management and analysis limitations, as well as the scale of the problems to be addressed, require these broad-scale efforts to assume uniform average habitat conditions and/or species assemblages or populations within stream reaches (McKenna et al. 2006, Sowa et al. 2007, Kanno et al. 2014). Stream reaches (in the Great Lakes Region) are typically small (< 3 km) rarely exceeding 10 km. They may be clearly defined on a map by upstream and downstream confluences and are often the minimum reporting units for broad scale analyses. The National Hydrography Database (NHD) is widely used as a geographic foundation of these analyses and is an effective information management and analysis tool, allowing for association of local and broad-scale habitat and biological information with each stream reach within a region or the entire nation (Huang and Frimpong 2016). The NHD stream network consists of confluence-to-confluence stream reaches that range widely in length and vary with spatial resolution (e.g., 1: 100,000 or 1: 24, 000).
It is well known that there is variability in both habitat and species assemblages within and between streams (Fischer and Parukert 2009, Espirito-Santo et al. 2013, Espirito-Santo. 2014, Longo and Blanco 2014, Miyazono and Taylor 2013). Fish assemblages typically become more diverse as stream size increases and assemblage composition often changes by addition of species to the upstream pool (Sheldon 1968). These changes are associated with a variety of conditions, including but not limited to, water temperature and groundwater input, stream and drainage size, flow hydrodynamics, water chemistry, and land use (Sheldon 1968, Taylor 1997, Matthews 1998, Lamouroux and Souchon 2002, McKenna 2005, Hughes et al. 2006, Gido and Jackson 2010, Kanno et al. 2014). Variability of habitat conditions, particularly flow, and position within the drainage network also affect fish assemblages (Horwitz 1978, Schlosser 1985, Poff and Allan 1995, Taylor 1997, Grossman and Sabo 2010). First- and second-order streams are the most numerous within any lotic system and the assumption of uniform conditions or assemblages may be least accurate in these headwater streams, because species and habitat diversity are low, habitat volume is small, and variability is high (Sheldon 1968, Horwitz 1978, Schlosser 1985, Poff and Allan 1995, Matthews 1998).
Many comparisons of species assemblages and habitat conditions have been made between streams, but few studies have reported intra-stream reach assemblage differences (Hughes et al. 2006, Gido and Jackson 2010, Espirito-Santo et al. 2013, Kanno et al. 2014, Longo and Blanco 2014, Longo and Blanco 2014, Miyazono and Taylor 2015). A few of these have noted greater habitat condition variability between streams than within stream reaches. Evaluation of aquatic species assemblage structure within headwater stream reaches is needed to determine the validity of within stream uniformity. To initially address this need, I examined habitat and local fish assemblages within single reaches of two headwater streams of the Oswego River Watershed, New York, USA, for evidence of within and between stream reach fish assemblage structure.
METHODS
Field-site Description
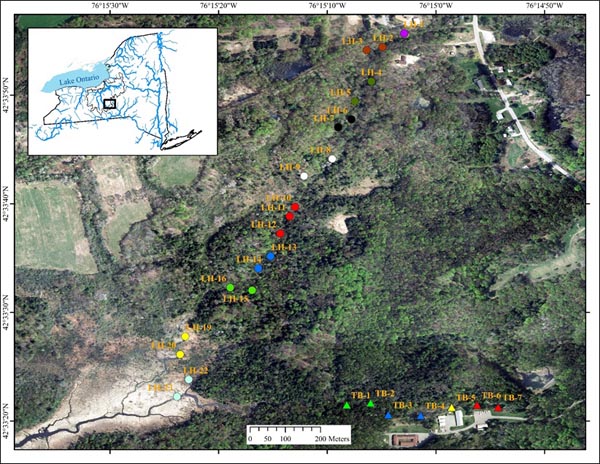
Tunison Brook (TB) and Lime Hollow Creek (LH) are headwaters streams in adjacent valleys of the Oswego River Watershed, which empties into eastern Lake Ontario (Fig. 1). Tunison Brook is a first order stream reach ~450 m long, draining a ~94.4 ha local watershed at the US Geological Survey, Tunison Laboratory of Aquatic Science (TLAS) and flowing through hemlock and deciduous forest, with a small fishless pond at its head and a small wooded wetland area in the middle. Lime Hollow Creek is a second order stream reach ~1,500 m long, draining a ~244.5 ha local watershed and flowing through mixed forest of the Lime Hollow Center for Environment and Culture for most of its length (~1,150 m), but with a marshy wetland delta (~300 m) at its mouth and a small pond (fish present) at its head.
Field Collections
Fish collections were made progressively throughout the length of the forested portions of each stream reach, from mid-August - mid-October 2011. Each site was separated by at least 30 m and collections proceeded from downstream to upstream to minimize disturbance to subsequent sites. Four additional samples were collected in late September 2012 within the downstream wetland habitat of the LH stream reach. We used a standard 50-m one-pass electrofishing method with a blocking seine at the upstream end to collect fish assemblages (McKenna and Johnson 2005). All fish were identified, counted, and recorded, and then released alive, except for those that were required as voucher specimens to verify identification. Each species was assigned to a feeding group according to Halliwell et al. 1999) to examine functional components of distinct assemblages. A suite of local habitat data including, water temperature, specific conductivity, dissolved oxygen (DO), pH, stream width, water depth, and discharge were collected at each site.
Statistical Analyses
In addition to detailed species composition, the total number of fish collected, the species richness (number of different species), the species diversity (H’, Shannon and Weaver 1959), and assemblage evenness (Pielou 1977) were computed for each sample assemblage. Multivariate data sets contain a myriad of informational threads and bootstrapping cluster analysis is most appropriate for objectively identifying significantly different groups of multispecies assemblages (McKenna 2003). It was applied here to determine if distinct fish assemblages were present within and/or between the study stream reaches. Fish abundances were ln-transformed and the Bray-Curtis similarity index with a UPGMA linkage method used. Each linkage was tested for significance at the α = 0.05 level with 1000 bootstrap samples. Enhanced discriminatory power can be achieved by a priori groupings, but this can also introduce bias. Six different a priori pairings were used to help detect significant groups and to test for bias. Three different random pairings within each stream reach and one completely random regardless of stream reach were tested; these homogenize assemblages, but any strong intrareach community structure should persist. Longitudinally paired adjacent samples (those most likely to be autocorrelated) were also tested as replicate representatives of the local assemblages within each stream reach. One case consisted of consecutive pairs beginning with the most upstream sample pair. The other was quite similar, but considered apparent habitat breaks, where the first sample below the pond of Lime Hollow Creek (LH-1) was unique (unpaired with another sample), as was the site in the TB wooded wetland (TB-5), and the three geographically closest Lime Hollow Creek samples were grouped (LH-10, LH-11, and LH-12) (Fig. 1); all others were adjacent pairs beginning upstream.
One-way Analysis of Variance, followed by Tukey’s multiple comparison test, was used to determine significance of differences in mean values of habitat conditions and species assemblage characteristics between distinct assemblages identified by cluster analysis. The α = 0.05 significance level was used.
RESULTS
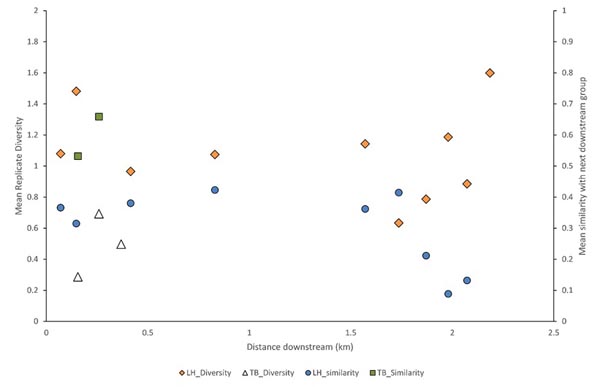
Fish and habitat conditions were collected from 27 sites, 7 from TB, and 20 from LH (Table 1) Fig. (1). A total of 2,921 fish were collected, with 2,668 from LH and 253 from TB; two of the TB sites were above a small barrier and had no fish. Sample fish abundance ranged 12-84 at TB (where fish were present) and 63-284 at LH. Sample richness was only 2-6 species at TB, but was as high as 13 at LH sites. The mean diversity of each replicate group of sample assemblages and similarity of adjacent assemblages (immediate downstream neighbor) varied widely and showed no clear trend, although similarity declined at the most downstream LH sites (Fig. 2).
Distinct Assemblages
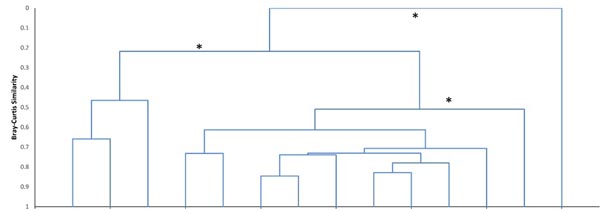
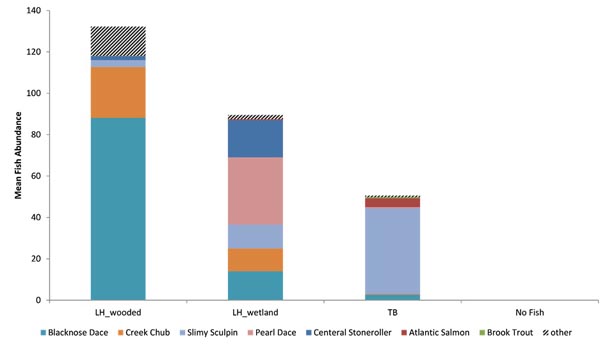
Cluster analysis showed the variation in local fish assemblages within each stream reach, but the bootstrapping test did not detect significant community structure with any of the randomly paired data sets. However, analysis of geographically grouped data revealed three distinct fish assemblages, plus the No Fish group in the upper most portion of the TB reach (Fig. 3). The same result occurred with either geographic replicate grouping. All TB sample assemblages (where fish were present) ranged in similarity from 0.46 to 0.66, but formed one group (TB) and all LH assemblages (LH_wooded; similarity range: 0.61 - 0.84), except the two most downstream wetland sites (LH_wetland) formed a distinct group. The TB assemblage was dominated by Slimy Sculpin (Cottus cognatus R.), Atlantic Salmon (Salmo salar L.), and Blacknose Dace (Rhinichthys atratulus H.), while the LH_wooded assemblage was dominated by Blacknose Dace and Creek Chub (Semotilus atromaculatus M.). In the LH_wetland assemblage, dominance was more evenly distributed among Pearl Dace (Margariscus margarita C.), Central Stoneroller (Campostoma anomalum R.), Blacknose Dace, Slimy Sculpin, and Creek Chub. Both alpha diversity and evenness were greatest in the LH_wetland assemblage (H’ = 1.6, V = 0.5), relatively high in the LH_wooded assemblage (H’ = 1.0, V = 0.4), and low in the TB assemblage (H’ = 0.5, V = 0.2) (LH_wetland-LH_wooded: p = 0.015, LH_wetland-TB: p = 0.001, and LH_wooded-TB: p = 0.038). Other differences also existed between the two adjacent stream reaches. Overall fish abundance was greater in the LH_wooded assemblage (132) than the TB assemblage (51), but variability was too great for 95% confidence (p = 0.06) (Table 1, Fig. 4). Alpha species richness was greater in the LH_wooded assemblage (8.3) than in the TB assemblage (3.6) (p < 0.01).
Species composition varied, but four species were present in all assemblages, Blacknose Dace, Creek Chub, Pearl Dace, and Slimy Sculpin (Table 2, Fig. 4). Eleven species were unique to the LH_wooded assemblage Blacknose Shiner (Notropis heterolepis E.), Brown Bullhead (Ameiurus nebulosus L.), Fathead Minnow (Pimephales promelas R.), Golden Shiner (Notemigonus crysoleucas M.), Largemouth Bass (Micropterus salmoides L.), Common Shiner (Luxilus cornutus M.), Pumpkinseed (Lepomis gibbosus L.), Rock Bass (Ambloplites rupestris R.), Spotfin Shiner (Cyprinella spiloptera C.), Tessellated Darter (Etheostoma olmstedi S.), and White Sucker (Catostomus commersonii T.). Blacknose Dace were significantly more abundant in the LH_wooded assemblage than in the TB assemblage (p = 0.002). The LH_wetland assemblage had greater abundances of Pearl Dace (p < 0.001) and Central Stoneroller (p = 0.001) than in the other assemblages. The TB assemblage was dominated by Slimy Sculpin with small components as Atlantic Salmon and Brook Trout (Salvelinus fontinalis M.). Slimy Sculpin was more abundant than in the other (p < 0.02) assemblages and Atlantic Salmon was more abundant than in the LH_wooded (p = 0.011) assemblage. Only Rainbow Trout (Oncorhynchus mykiss W.) was unique to the TB assemblage.
| Assemblage | Site code | N | S | H | V |
|---|---|---|---|---|---|
| LH_wooded | LH-1 | 108 | 9 | 1.08 | 0.35 |
| LH-2 | 284 | 8 | 1.23 | 0.40 | |
| LH-3 | 121 | 13 | 1.73 | 0.56 | |
| LH-4 | 115 | 6 | 0.67 | 0.22 | |
| LH-5 | 90 | 7 | 1.26 | 0.41 | |
| LH-6 | 108 | 9 | 1.18 | 0.38 | |
| LH-7 | 149 | 7 | 0.97 | 0.31 | |
| LH-8 | 160 | 11 | 1.13 | 0.37 | |
| LH-9 | 119 | 10 | 1.15 | 0.37 | |
| LH-10 | 63 | 6 | 0.40 | 0.13 | |
| LH-11 | 80 | 7 | 0.63 | 0.20 | |
| LH-12 | 85 | 6 | 0.87 | 0.28 | |
| LH-13 | 74 | 6 | 0.90 | 0.29 | |
| LH-14 | 90 | 8 | 0.68 | 0.22 | |
| LH-15 | 88 | 10 | 1.10 | 0.35 | |
| LH-16 | 97 | 9 | 1.28 | 0.41 | |
| LH-19 | 245 | 9 | 0.79 | 0.26 | |
| LH-20 | 305 | 8 | 0.98 | 0.32 | |
| Mean: | 132.3 ± 16.99 | 8.3 ± 0.45 | 1.00 ± 0.07 | 0.32 ± 0.02 | |
| TB | TB-1 | 84 | 4 | 0.38 | 0.12 |
| TB-2 | 71 | 4 | 0.62 | 0.20 | |
| TB-3 | 40 | 2 | 0.32 | 0.11 | |
| TB-4 | 46 | 6 | 1.06 | 0.34 | |
| TB-5 | 12 | 2 | 0.29 | 0.09 | |
| Mean: | 50.6 ± 12.56 | 3.6 ± 0.75 | 0.53 ± 0.14 | 0.17 ± 0.05 | |
| LH_wetland | LH-22 | 26 | 6 | 1.63 | 0.53 |
| LH-23 | 153 | 7 | 1.57 | 0.51 | |
| Mean: | 89.5 ± 63.5 | 6.5 ± 0.5 | 1.60 ± 0.03 | 0.52 ± 0.01 |
| Species | LH_wooded | LH_wetland | TB |
|---|---|---|---|
|
Atlantic Salmon (P) Salmo salar |
0.00 ± 0.00 [I] | 0.50 ± 0.50 [I-II] | 4.20 ± 2.58 [II] |
|
Blacknose Dace (I) Rhinichthys atratulus |
88.06 ± 11.35 [I] | 14.00 ± 8.01 [I-II] | 2.60 ± 2.60 [II] |
|
Blacknose Shiner (I) Notropis heterolepis |
0.28 ± 0.28 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Brook Trout (P) Salvelinus fontinalis |
0.06 ± 0.06 | 0.00 ± 0.00 | 0.60 ± 0.40 |
|
Brown Bullhead (O) Ameiurus nebulosus |
0.17 ± 0.12 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Brown Trout (P) Salmo trutta |
0.44 ± 0.19 | 0.00 ± 0.00 | 0.40 ± 0.40 |
|
Central Stoneroller (H) Campostoma anomalum |
1.94 ± 0.45 [I] | 18.00 ± 16.02 [II] | 0.00 ± 0.00 [I] |
|
Common Shiner (I) Luxilus cornutus |
0.67 ± 0.26 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Creek Chub (I) Semotilus atromaculatus |
24.67 ± 5.76 | 11.00 ± 7.01 | 0.40 ± 0.25 |
|
Cutlip Minnow (I) Exoglossum maxillingua |
1.50 ± 0.38 | 2.00 ± 1.00 | 0.00 ± 0.00 |
|
Fathead Minnow (I) Pimephales promelas |
2.39 ± 1.57 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Golden Shiner (I) Notemigonus crysoleucas |
0.17 ± 0.12 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Largemouth Bass (P) Micropterus salmoides |
0.89 ± 0.30 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Pearl Dace (O) Margariscus margarita |
0.06 ± 0.06 [I] | 32.50 ± 27.54 [II] | 0.60 ± 0.40 [I] |
|
Pumpkinseed (I) Lepomis gibbosus |
0.50 ± 0.25 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Rainbow Trout (P) Oncorhynchus mykiss |
0.00 ± 0.00 [I] | 0.00 ± 0.00 [I-II] | 0.40 ± 0.25 [II] |
|
Rock Bass (P) Ambloplites rupestris |
0.11 ± 0.11 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Slimy Sculpin (I) Cottus cognatus |
3.22 ± 0.66 [I] | 11.5 ± 3.51 [II] | 41.4 ± 11.51 [II] |
| Spotfin Shiner (I) Cyprinella spiloptera |
0.39 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
Tessellated Darter (I) Etheostoma olmstedi |
2.39 ± 0.59 | 0.00 ± 0.00 | 0.00 ± 0.00 |
|
White Sucker (O) Catostomus commersonii |
4.39 ± 1.73 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Cluster | LH_wooded | LH_wetland | TB | No Fish |
|---|---|---|---|---|
| Temperature (°C) | 15.89 ± 0.31 (I) | 11.86 ± 0.49 (II) | 10.80 ± 0.04 (II) | 10.19 ± 0.06 (II) |
| Conductivity (µS/cm) | 463 ± 1.74 (I) | 445 ± 0.00 (I) | 481 ± 0.00 (II) | 512 ± 8.58 (III) |
| DO (% saturation) | 99.66 ± 1.07 (I) | 109.40 ± 1.20 (II) | 96.52 ± 2.05 (I) | 96.65 ± 0.66 (I) |
| DO (mg/l) | 9.86 ± 0.17 (I) | 11.82 ± 0.01 (II) | 10.68 ± 0..22 (I-II) | 10.85 ± 0.08 (I-II) |
| pH | 8.81 ± 0.02 (I) | 9.21 ± 0.13 (II) | 9.17 ± 0.03 (II) | 8.84 ± 0.17 (I-II) |
| Width (m) | 4.2 ± 0.3 (I-II) | 3.4 ± 0.8 (I-II) | 5.2 ± 0.0 (I) | 1.8 ± 0.71 (II) |
| Depth (cm) | 20.7 ± 1.9 (I-II) | 29.6 ± 12.8 (I) | 12.3 ± 3.4 (I-II) | 7.1 ± 1.2 (II) |
| Discharge (m3/s) | 0.3257 ± 0.0249 (I) | 0.1290 ± 0.0894 (I-II) | 0.0012 ± 0.0001 (II) | 0.0001 ± 0.0003 (II) |
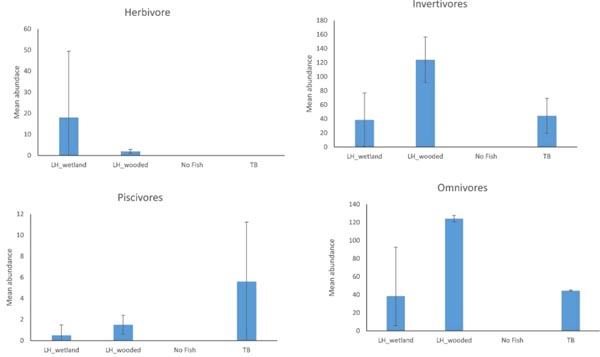
The abundances of functional groups also varied among distinct assemblages (Fig. 5). Herbivores were represented by only Central Stonerollers and were most abundant in the LH_wetland assemblage. Six species of piscivores were present in the data and their abundance was greatest in the TB assemblage. However, both of these groups varied widely. Invertivore and Omnivore abundances also varied, but were more abundant in the LH_wooded assemblage than in other assemblages.
Habitat Conditions
Local habitat conditions associated with each distinct assemblage also differed in some ways (Table 3). The LH_wooded assemblage habitat conditions had lower pH (p = 0.003) and higher discharge (p < 0.001) than the TB assemblage. That assemblage was also warmer (p < 0.001) than any of the other habitats and had greater discharge than the TB (p < 0.001) assemblage or No Fish habitat (p = 0.003). The LH_wetland assemblage was associated with greater DO (p = 0.02–0.04) than other habitats, higher pH (p = 0.035) than LH_wooded, and greater depth than the No Fish habitat. The No Fish habitat had the highest conductivity (p < 0.01) and the TB assemblage was associated with higher conductivity (p = 0.009) than either the LH_wooded or LH_wetland assemblages.
DISCUSSION
The results clearly identified a uniform assemblage within each of the study stream reaches, except for the distinct assemblage in the wetland section of the LH reach. This assemblage structure was not detected when samples were paired randomly, but existed only when spatially adjacent local assemblages were grouped. Thus, spatial autocorrelation contributed to fish community structure within the LH reach, and that structure was associated with substantial change in habitat. The diverse Blacknose Dace and Creek Chub dominated assemblage persisted throughout this LH reach, but was modified enough in the wetland habitat by Central Stoneroller, Pearl Dace, and Slimy Sculpin to make those assemblages distinct. Despite some variation in habitat conditions, the sculpin and salmonid dominated assemblage was found throughout the TB reach (where fish were present).
The LH_wetland group was distinct from the fish assemblage of the forested habitat upstream, likely due to differences in habitat, but could also have resulted because the wetland samples were collected in a different year (although the same season). However, some aspects of local habitat conditions (e.g., water temperature, DO, and pH) clearly differed between the wetland and wooded habitats. Also, the two wetland samples closest to the forested habitat grouped with the LH_wooded assemblage, which suggests the assemblage differences are more likely related to habitat differences than temporal variation. Species composition may also indicate habitat related differences. For example, abundant Pearl Dace (omnivore) and Central Stoneroller (herbivore) set the LH_wetland assemblage apart from the others. Pearl Dace are often associated with wetlands and will feed on bivalves and algae (Scott and Crossman 1973), (Johnson and Johnson 1982). Central Stonerollers feed by scraping algae and invertebrates from hard surfaces, which may be more abundant in open, wetland habitat than wooded habitat.
Functional component differences of the distinct assemblages reflect both the changes associate with a shift in land cover and differences between streams. Similarly, lack of a downstream trend in alpha diversity or similarity, except near the wetland component of the LH stream, supports the uniformity of fish assemblages within these stream reaches, but with evidence of the effect of land use change (forest to wetland). Thus, assemblage uniformity may depend upon homogeneity of land cover along the stream reach or throughout its local watershed.
The literature includes numerous examples of longitudinal changes in fish assemblage structure within a watershed (Sheldon 1968, Horwitz 1978), (Matthews 1998), Bistoni and Hued 2002, for example) and differences between streams and watersheds (Valerio et al. 2007, Guimaraes et al. 2010, Tondato and Suarez 2010, for example). However, examination of the literature failed to yield other studies that have reported within stream reach species assemblage structure. In an examination of fragmented pools in a Texas stream, Miyazono and Taylor (2015) found fish assemblages in shallow pools were nested subsets of those in larger pools. However, they did not report the similarity among assemblages within each reach or pool. Similarly, Fischer and Paukert (2009) collected nested samples of different effort (distances shocked) to determine effort needed to detect different assemblages between streams, but did not examine differences in local assemblages within streams. Espirito-Santo et al. (2013) also examined fish assemblages at fine-scales within Amazonian headwater streams and were able to identify microhabitat associations and movements between the river and floodplain, but they did not discuss the similarity of assemblages within each reach. Gido et al. (2006) examined fish assemblages in prairie streams of Kansas at three different spatial scales (including the site scale) and determined the relative influence of regional and reach scale variables on assemblage structure, but did not specify the similarity of assemblages at sites within reaches. Taylor (1997) was able to show that the species richness and nestedness of isolated and connected pools within a reach (11 km) of an Oklahoma stream responded differently to habitat volume and distance from source populations. However, the similarity among pool assemblages was not explicitly reported.
This study was limited spatially and temporally. These results apply to headwaters, which are most numerous, and collectively drain the largest portion of a watershed. However, higher order streams have more habitat volume, and are typically more stable and productive than small streams (Vannote et al. 1980, (Schlosser 1987, Roberts and Hitt 2010). Uniform species assemblage structure is unlikely to persist there. Even in some headwaters, fish arrange themselves according to microhabitats (Espirito-Santo et al. 2013, Johnson and Chalupnicki 2014), which may be the result of species specific morphological adaptations (Arnold et al. 1991). The results indicate that substantial changes in land cover can affect fish assemblages within stream reaches. However, samples for this study came from healthy forested habitats with only small components as wetland. Fish assemblages within different land use types, such as agricultural or urban, may exhibit different within stream reach assemblage structure. This research was also limited to only summer and early fall, but fish assemblage structure may differ in other seasons. It is well known that spring assemblages can change due to influx of migratory species.
Inter-reach Contrasts
Despite the uniformity of local species assemblages within these adjacent stream reaches, there were clear differences between them. The greater diversity of the LH_wooded assemblage (second order stream) than the TB assemblage (first order stream) is consistent with the known pattern of increased diversity with stream size or drainage area (Sheldon 1968), (Horwitz 1978), (Allan 1995, Matthews 1998). Other streams in the same region as this study were also found to have similar diversity and species composition of headwater stream fish assemblages (Sheldon 1968).
There is often evidence of metacommunity dynamics and support of local tributary or pool assemblages by movement of fish from larger pools of species in separate downstream reaches (Horwitz 1978, Miyazono and Taylor 2015, for example). However, the LH assemblages and TB assemblages were only 20% similar despite being connected at their confluence (Fig. 3). Thus, internal structuring forces (e.g., distinct habitat conditions) seem to be more important than the influence of dispersal, in these streams.
CONCLUSION
Variability increases as scale decreases, thus the amount of data and frequency at which data must be updated increases rapidly at fine scales. Even with modern data collection and processing technology, analyzing nationwide data at very fine scales can be prohibitive. However, important broad-scale ecological questions can be addressed if generalizations of fine-scale conditions do not substantially affect the conclusions. First and second-order headwater streams are most numerous in any lotic system and clearly have different habitat conditions that may support different aquatic assemblages, but simplified, average representation of reach level fish assemblages allows for effective coarser scale evaluations. While the results of this study support the general assumption that fish assemblages are uniform within stream reaches, for the purposes of coarse scale analyses, only two streams were examined here and more research is needed to test the consistency of these finding across all headwater streams of a watershed or region.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
I am grateful to M. Chalupnicki for assistance with field sampling and data management. I appreciate field collection assistance rendered by G. Riesweber, P. Harrity, and other volunteers. I would like to thank the Lime Hollow Center for Environment and Culture for its cooperation with this study. My thanks go to the reviewers of this paper. This is a contribution of the US Geological Survey, Great Lakes Science Center. Animal Care and Use protocols followed.
REFERENCES
[CrossRef Link]
[CrossRef Link] [PubMed Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]
[CrossRef Link]


